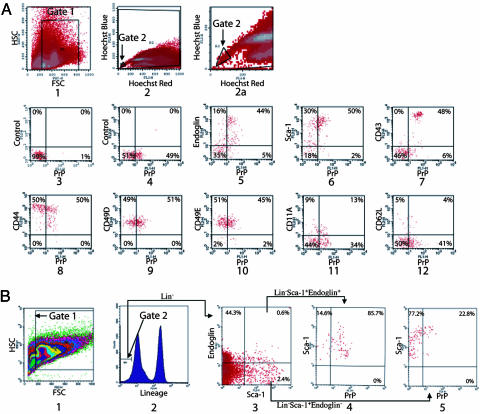
Fig. 1.
PrP is expressed on bone marrow populations enriched in HSC activity. (A) Freshly isolated BM cells were stained with Hoechst dye 33342, and the SP fraction was gated (gates 1 and 2) to analyze the expression of PrP. In plot 1, forward scatter (FSC) and side scatter (SSC) is used to gate on hematopoietic cells. Hoechst Red and Hoechst Blue (plots 2 and 2a, gate 2 was set as 0.02% of total cells) represent two fluorescence emission wavelengths used to detect the SP cells. Plot 2a is an expansion of the gate 2 region of plot 2. PrP-null BM SP cells served as a negative control for PrP antibody staining (plot 3). WT BM SP cells were stained for PrP (plots 4–12) together with isotype control (plot 4), Endoglin (plot 5), Sca-1 (plot 6), CD43 (plot 7), CD44 (plot 8), CD49D (plot 9), CD49E (plot 10), CD11A (plot 11), or CD62L (plot 12). (B) Total BM cells were stained with anti-Endoglin followed sequentially by anti-rat-PE/CY5.5, a mixture of biotinylated lineage-specific antibodies, and streptavidin–APC, anti-PrP-FITC, and anti-Sca-1-PE. Plot 1 shows the gate of FSC and SSC channels. The lowest 5% of APC-stained cells (i.e., Lin−) were gated (plot 2). Plot 3 shows the staining of the gated Lin− cells with Sca-1 and Endoglin, and plot 4 shows the staining of the gated Lin− Sca-1+ Endoglin+ cells with PrP. Plot 5 shows the staining of the gated Lin− Sca-1+ Endoglin− cells with PrP.