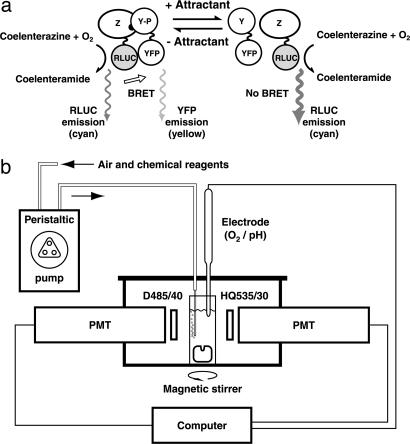
Fig. 1.
Principle of BRET and apparatus for in vivo measurement. (a) BRET between RLUC and YFP can be used to measure interactions between a pair of proteins to which they are fused genetically. In the chemotaxis pathway, the affinity between CheZ and CheY increases with CheY phosphorylation, so pathway activity can be monitored by measuring BRET between CheZ–RLUC and YFP–CheY. In the absence of attractant (Left), phosphorylated CheY (CheY-P) is relatively abundant, so BRET from RLUC to YFP is efficient and yellow emission is relatively large. Upon addition of attractant (Right), rapid dephosphorylation of CheY-P results in decreased BRET and YFP emission, so the spectrum is shifted toward the blue. (b) Bioluminescence from a stirred suspension of swimming cells was measured in a cuvette placed in a light-tight box, as described in Methods. A peristaltic pump delivered air to the sample and the other reagents required for the experiments. The two photomultiplier tubes (PMTs) collected light through bandpass filters mounted between their photocathodes and the cuvette (D485/40 and HQ535/30, for the cyan and yellow channels, respectively). The PMT output signals were analyzed online by a computer. In some experiments, an optional electrode for monitoring oxygen or pH was inserted into the cuvette through a hole in the lid of the box, which otherwise remained sealed.