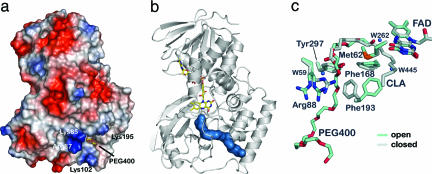
Fig. 3.
Substrate entry channel and gating mechanism in PAI. (a) The surface potential of PAI shows an electropositive area localized at the entrance of the channel that is created by Lys-85, Arg-87, Lys-102, and Lys-195. The PEG 400 molecule marks the entry of the channel. (b) The molecular surface (blue) of part of the PEG 400 molecule bound to PAI in the absence of substrate/product shows the 30-Å path from the surface to the active site FAD (drawn as sticks). (c) Conformational changes in active site associated with PEG 400 binding reveal the gating mechanism. PEG 400, Phe-193, and Arg-88 are in the open conformation when PEG 400 is bound (blue) compared with the apoenzyme (gray). Arg-88 displays two conformations in the open form of PAI, both of which point away from the entering substrate.