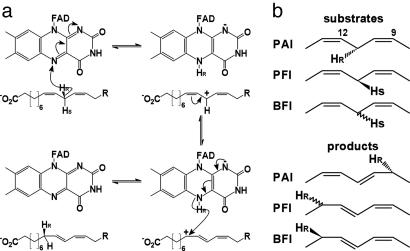
Fig. 4.
Stereochemistry of PAI reaction. (a) Structure-based isomerization mechanism of LA to 10,12-CLA. Pro-R Hydride abstraction by FAD atom N5 yields an intermediate LA carbocation, which is stabilized by π-conjugation. Hydride transfer to the Re-side of LA atom C9 yields 10,12-CLA and regenerates the oxidized form of FAD. (b) Stereochemistry of hydrogen transfer in PUFA isomerases. In PAI the hydride is abstracted from and transferred back to the fatty acid from the Re-side. For PFI, abstraction from the Si-side was reported, but no information is available for the back-transfer of the H-atom. The situation is reversed in BFI, where the abstraction face is unknown, but the H-atom is transferred back from the Re-side.