A crucial requirement of metabolically active aerobic cells is a steady supply of oxygen. The red pigment of vertebrate skeletal muscle, oxygen-binding myoglobin (Mb), serves this function by facilitating the delivery of O2 from the plasma membrane to the energy-producing mitochondria (1). The delivery of O2 from lungs or gills to muscles is also very efficient because of the cooperative loading and unloading of O2 by the hemoglobin of the red cells. Since Ray Lankester’s (2) first identification, vertebrate Mb has been believed to occur solely in cardiac and skeletal muscle. Only one exception was noted: the smooth muscle sphincter cells of the human rectum, since confirmed (3). Mb can act as a store of O2 to help maintain a constant supply of O2 during rapidly fluctuating demands of contraction (4). This helps explain why the concentration of Mb is highest in the skeletal muscles of diving mammals, and why Mb increases in animals, including humans, after chronic muscular activity or hypoxia (4, 5). In human skeletal muscle, Mb in mitochondria-rich oxidative myofibers shows elevated synthesis in response to exposure to high altitudes (5) or intense endurance training under reduced oxygen pressures (4–6). Fraser et al. (7) now report, on page 2977 in this issue of PNAS, that the hypoxia-tolerant common carp (Cyprinus carpio) has Mb not only in muscle but also in other metabolically active tissues that include liver, brain, and gills.
What is Mb doing in these nonmuscle tissues? Fraser et al. (7) have addressed this question by identifying two unique Mbs in carp tissue, Myg-1 and -2. Myg-1 is expressed not only in muscle but also in liver, kidney, and gill tissue. In these tissues, the Myg-1 gene is strongly up-regulated in hypoxia. For example, Myg-1 mRNA expression in liver increased 20-fold after 5 days of hypoxia, and the protein increased 2- to 3-fold to approximately half the quantity found in skeletal muscle of nondiving mammals (4). In contrast, expression of Myg-2 occurred only in brain tissue, where it was independent of changes in oxygen pressure. This difference in gene control strongly suggests different functions for the two carp Mbs.
Robust in vivo induction of Mb genes by chronic hypoxia, regardless of the training status of the animals, was previously observed in zebrafish gills (8) and in striated muscle of mice (9). The master regulator of cellular O2 homeostasis, the hypoxia-inducible transcription factor 1 (HIF-1), has been implicated in the mouse for this transcriptional activation (9). The possibility that HIF-1 might generally be involved in the induction of Mb is supported by two observations. First, stimulation of the HIF-1 pathway in muscle is triggered by exercise with or without hypoxia and mechanical stress (stretching) (6, 10, 11). Second, the HIF-1/Mb system is colocalized in oxidative skeletal myofibers (type I and IIA fibers) (4, 12). Posttranscriptional controls might also participate in the hypoxic stimulation of some Mbs, because several hypoxia-inducible mRNA stabilization signals have been discovered in the 3′ untranslated regions of human and rodent sequences (13). Therefore, the link between Mb synthesis and exercise (with and without hypoxia), along with possible regulation via HIF-1-dependent and independent mechanisms, can reasonably be extended to carp.
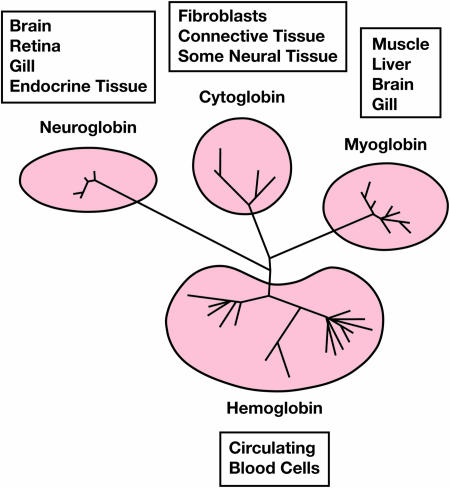
The presence of a unique Mb in fish brain and another in muscle, liver, kidney, and gill tissue adds to the growing inventory of oxygen-binding tissue globins. These include unique neuroglobins (Ngb) in mammalian neural and endocrine tissue and cytoglobins (Cygb), found primarily in fibroblast-like cells of various organs (e.g., heart, liver, colon, and kidney) (14). The Fraser study (7) contributes to this bewildering complexity (Fig. 1) by reporting that carp brain also expresses Ngb transcripts in addition to Myg-2. On the other hand, Carp Cygb shows an unusual, hypoxia-unresponsive expression pattern that is in contrast to the generally inducible synthesis of mammalian Cygb (9, 15).
Fig. 1.
Tissue distribution and phylogeny of fish globins. The phylogenetic tree has been adapted from Fraser et al. (7).
What are all of these oxygen-binding heme proteins doing? Hankeln et al. (14) note a close correlation between the concentration of Ngb and metabolic activity. For example, the mammalian retina has one of the highest rates of oxygen consumption of any tissue, and the concentration of Ngb, ≈100 μM, is comparable to that of Mb in skeletal muscle. The Ngb appears to mediate the delivery of oxygen to the mitochondria in the photoreceptor cells. Fraser et al. (7) find that 3 days of exposure to hypoxia (≈10% normoxia) resulted in a 4-fold increase in mRNA expression of Myg-1 in carp muscle but only at the stressful temperature of 30°C. Hypoxic exposure of fish at 17°C yielded no Myg-1 elevation in muscle at all. This finding is not entirely unexpected. Lower-body temperatures are used by many ectotherms to reduce oxygen consumption and metabolic rate (16). In contrast, hypoxia-induced expression of Myg-1 mRNA in liver increased 20-fold in 3 days at 30°C. Brain tissue, however, showed no significant hypoxiadependent change. Do the differing responses to hypoxia in the various tissues reflect different functions for Mb?
The answer may be yes. Mb may function in ways not directly related to cellular oxygenation. This is suggested by mice engineered to lack Mb. They have normal reproductive and exercise capacity and skeletal and cardiac muscle function (17, 18). It is unclear whether both the brain Ngb and the brain Mb, Myg-2, occur within the same cells in the carp, but it is of interest that neither protein is strongly influenced by hypoxia. This suggests that additional nonoxygen transport functions are likely. One such function is participation in nitric oxide (NO) metabolism and homeostasis (19, 20). Mb binds not only O2 but also NO. Ferrous Mb reacts with NO in the presence of O2 to produce ferric Mb and nitrate:
. The Fe3+Mb so formed is quickly removed by metMb reductase (21). Such a scavenging function of Mb for NO (19) is supported by the finding that hearts isolated from Mb-null mice are more strongly impaired by excess NO than are wild-type hearts (18). Excess NO, produced by treatment with bradykinin, which up-regulates NO synthase, competes with O2 for cytochrome c oxidase and so inhibits respiration. Brunori (20) points out that Mb would protect the cytochrome c oxidase from this insult by destroying the NO.
The remarkable discovery by Fraser et al. (7) of Mb in nonmuscle tissue immediately raises several questions. Is this finding confined to fish? Does it correlate with hypoxia tolerance in aquatic organisms? It is as yet unknown whether Mb in nonmuscle tissue occurs in any terrestrial vertebrate or amphibian. Just as the extent of nonmuscle Mb is unclear, so are the detailed functions of these nonmuscle globins: Mbs, Cygbs, and Ngbs. We speculate that the function of Mb and other globins in these tissues may involve NO, but several other possibilities exist. The diverse distribution and function of invertebrate and microbial hemoglobins (22, 23) suggest that some vertebrate globins might have similar functions, such as that of a dioxygenase, oxygen sensor, or terminal oxidase. Clearly, the discovery of the new Mbs, Ngbs, and Cygbs opens a rich field for future studies.
Conflict of interest statement: No conflicts declared.
See companion article on page 2977.
References
- 1.Wittenberg J. B., Wittenberg B. A. J. Exp. Biol. 2003;206:2011–2020. doi: 10.1242/jeb.00243. [DOI] [PubMed] [Google Scholar]
- 2.Lankester E. R. Pflügers Arch. Ges. Physiol. 1871;4:315–320. [Google Scholar]
- 3.Qiu Y., Sutton L., Riggs A. F. J. Biol. Chem. 1998;273:23426–23432. doi: 10.1074/jbc.273.36.23426. [DOI] [PubMed] [Google Scholar]
- 4.Ordway G. A., Garry D. J. J. Exp. Biol. 2004;207:3441–3446. doi: 10.1242/jeb.01172. [DOI] [PubMed] [Google Scholar]
- 5.Reynafarje B. J. Appl. Physiol. 1962;17:301–305. doi: 10.1152/jappl.1962.17.2.301. [DOI] [PubMed] [Google Scholar]
- 6.Vogt M., Puntschart A., Geiser J., Zuleger C., Billeter R., Hoppeler H. J. Appl. Physiol. 2001;91:173–182. doi: 10.1152/jappl.2001.91.1.173. [DOI] [PubMed] [Google Scholar]
- 7.Fraser J., de Mello L. V., Ward D., Rees H. R., Williams D. R., Fang Y., Brass A., Gracey A. Y., Cossins A. R. Proc. Natl. Acad. Sci. USA. 2006;103:2977–2981. doi: 10.1073/pnas.0508270103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Meer D. L., van den Thillart G. E., Witte F., de Bakker M. A., Besser J., Richardson M. K., Spaink H. P., Leito J. T., Bagowski C. P. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R1512–R1519. doi: 10.1152/ajpregu.00089.2005. [DOI] [PubMed] [Google Scholar]
- 9.Fordel E., Geuens E., Dewilde S., De Coen W., Moens L. IUBMB Life. 2004;56:681–687. doi: 10.1080/15216540500037406. [DOI] [PubMed] [Google Scholar]
- 10.Ameln H., Gustafsson T., Sundberg C. J., Okamoto K., Jansson E., Poellinger L., Makino Y. Faseb J. 2005;19:1009–1011. doi: 10.1096/fj.04-2304fje. [DOI] [PubMed] [Google Scholar]
- 11.Chang H., Shyu K. G., Wang B. W., Kuan P. Clin. Sci. (London) 2003;105:447–456. doi: 10.1042/CS20030088. [DOI] [PubMed] [Google Scholar]
- 12.Mason S. D., Howlett R. A., Kim M. J., Olfert I. M., Hogan M. C., McNulty W., Hickey R. P., Wagner P. D., Kahn C. R., Giordano F. J., et al. PLoS Biol. 2004;2:1540–1548. doi: 10.1371/journal.pbio.0020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wystub S., Ebner B., Fuchs C., Weich B., Burmester T., Hankeln T. Cytogenet. Genome Res. 2004;105:65–78. doi: 10.1159/000078011. [DOI] [PubMed] [Google Scholar]
- 14.Hankeln T., Ebner B., Fuchs C., Gerlach F., Haberkamp M., Laufs T. L., Roesner A., Schmidt M., Weich B., Wystub S., et al. J. Inorg. Biochem. 2005;99:110–119. doi: 10.1016/j.jinorgbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M., Gerlach F., Avivi A., Laufs T., Wystub S., Simpson J. C., Nevo E., Saaler-Reinhardt S., Reuss S., Hankeln T., et al. J. Biol. Chem. 2004;279:8063–8069. doi: 10.1074/jbc.M310540200. [DOI] [PubMed] [Google Scholar]
- 16.Wood S. C. Annu. Rev. Physiol. 1991;53:71–85. doi: 10.1146/annurev.ph.53.030191.000443. [DOI] [PubMed] [Google Scholar]
- 17.Garry D. J., Ordway G. A., Lorenz J. N., Radford N. B., Chin E. R., Grange R. W., Bassel-Duby R., Williams R. S. Nature. 1998;395:905–908. doi: 10.1038/27681. [DOI] [PubMed] [Google Scholar]
- 18.Gödecke A., Flögel U., Zanger K., Ding Z., Hirchenhain J., Decking U. K., Schrader J. Proc. Natl. Acad. Sci. USA. 1999;96:10495–10500. doi: 10.1073/pnas.96.18.10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flögel U., Merx M. W., Gödecke A., Decking U. K. M., Schrader J. Proc. Natl. Acad. Sci. USA. 2001;98:735–740. doi: 10.1073/pnas.011460298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunori M. Trends Biochem. Sci. 2001;26:21–23. doi: 10.1016/s0968-0004(00)01698-4. [DOI] [PubMed] [Google Scholar]
- 21.Livingston D. J., McLachlan S. J., La Mar G. N., Brown W. D. J. Biol. Chem. 1985;260:15699–15707. [PubMed] [Google Scholar]
- 22.Weber R. E., Vinogradov S. N. Physiol. Rev. 2001;81:569–628. doi: 10.1152/physrev.2001.81.2.569. [DOI] [PubMed] [Google Scholar]
- 23.Vinogradov S. N., Hoogewijs D., Bailly X., Arrcdono-Peter R., Guertin M., Gough J., Dewilde S., Moens L., Vanfleteren J. R. Proc. Natl. Acad. Sci. USA. 2005;102:11385–11389. doi: 10.1073/pnas.0502103102. [DOI] [PMC free article] [PubMed] [Google Scholar]