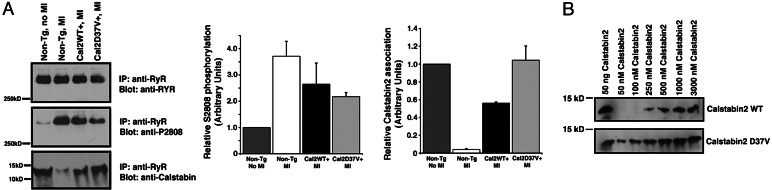
Fig. 7.
Increased expression of calstabin2 in the heart restores calstabin2 binding to PKA-phosphorylated RyR2. (A) Immunoprecipitated RyR2 complexes from heart lysates showed that WT calstabin2+ and calstabin2–D37V+ mice retained calstabin2 binding to the RyR2 complex despite PKA phosphorylation of RyR2. Densitometry was used to quantify the amount of PKA phosphorylation of RyR2–S2808 and the amount of calstabin2 bound to the RyR2 complex relative to non-Tg, noninfarcted mice. (B) Concentration curve of WT calstabin2 vs. calstabin2–D37V binding to PKA-phosphorylated RyR2 showed that, at physiologic levels of calstabin2, calstabin2–D37V exhibited increased binding to PKA-phosphorylated RyR2 compared with WT calstabin2. At high nonphysiological concentrations of WT calstabin2, binding to PKA-phosphorylated RyR2 was observed.