Figure 1. PKCɛ is an actin binding protein in lacrimal acini.
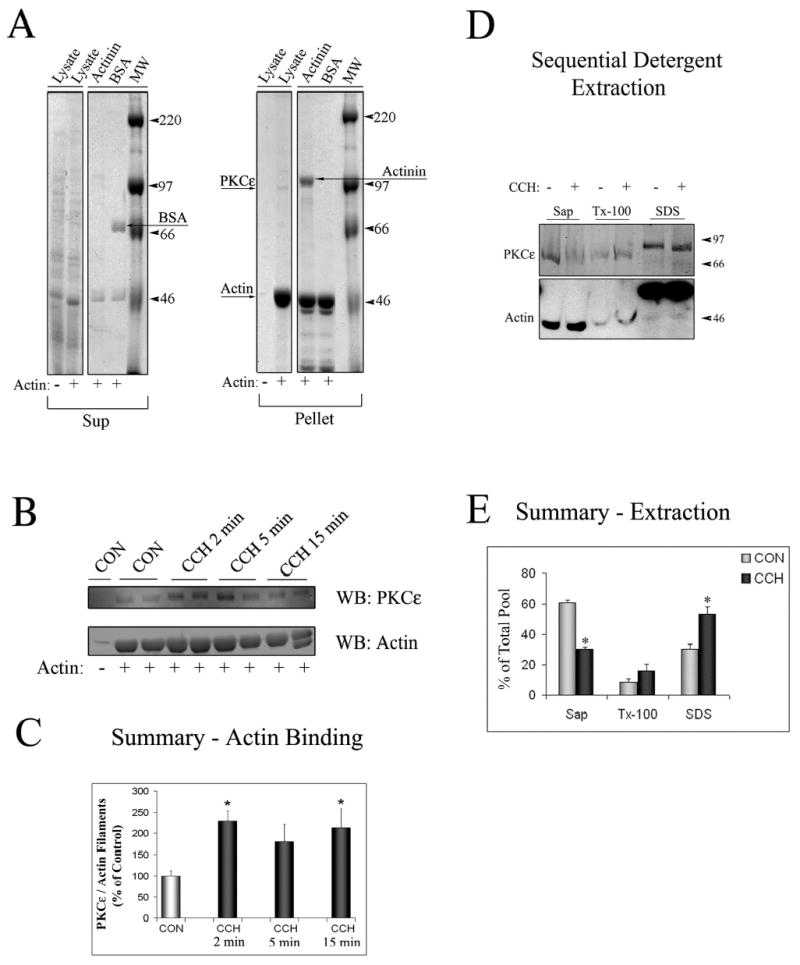
A. SDS-PAGE gel stained with Coomassie blue staining depicting the Supernatant (Sup) and Pellet fractions from actin-binding assays. Lysates from lacrimal acini (Lysate) were incubated without (−) or with (+) non-muscle actin and filaments were pelleted by centrifugation as described in Methods. α-Actinin (an actin-binding protein) and bovine serum albumin (BSA) were used as positive and negative controls, respectively. α-Actinin was pelleted with actin filaments (Arrow labeled Actinin showing its position in the Pellet fraction) while BSA was not (Arrow labeled BSA showing its position in the Sup fraction). A weaker protein signal showing a the major band with a MW corresponding to PKCɛ ~95kDa could also be detected in the Lysate lane in the Pellet fraction when non-muscle actin was added to the reaction (Arrow labeled PKCe). B. Western Blot analysis of the Pellet fraction from a representative actin-binding assay, when lysates (from equal amounts of cells) from acini stimulated without (CON) or with CCH for the indicated periods of time (2–15 min, 100 μM) were used for the actin binding assay and Pellet fractions were blotted for PKCɛ and actin as indicated. Duplicate samples were run in each assay as shown. C. Summary graph of actin-binding experiments in B. obtained from three independent preparations; *, significant at p ≤ 0.05. D. Acini without or with CCH (100 μM, 15 min) lacrimal acini were subjected to sequential detergent extraction to isolate soluble (Sol), membrane (Mem) and cytoskeletal (Cyt) pools as described in Methods. Equal volumes of each of the fractions were resolved by SDS-PAGE and the sample content of PKCɛ and actin determined by Western blotting. E. Composite values reflecting PKCɛ enrichment within soluble, membrane and cytoskeletal pools from acini without or with CCH as described in C. and expressed as a percentage of total cellular PKCɛ. Stimulation did not affect the recovery of marker proteins in the three fractions (data not shown). Results are from n=3 experiments; error bars represent sem; *, p ≤ 0.05.
