Fig. 5. Tyrosine phosphorylation in GAP motifs of the Kir3.4 N-terminal tail is the mechanism of trkB-mediated GAP activity.
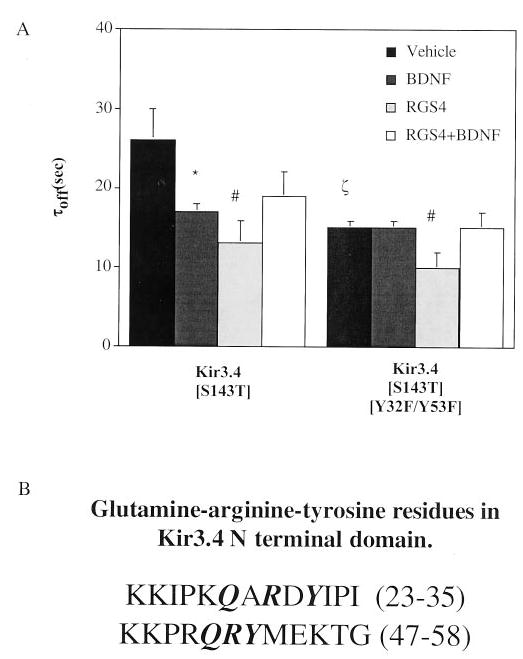
A, when tyrosines 32 and 53 were mutated to phenylalanines in Kir3.4(S143T) homomeric channels, channel deactivation was insensitive to trkB-mediated acceleration. Channel mutants were expressed in Xenopus oocytes, and two-electrode voltage clamp experiments were performed as described following pretreatment in vehicle or BDNF. Overexpression of 10 ng of RGS4 12–16 h prior to two-electrode voltage clamp recordings accelerated channel deactivation independently of Kir3 GAP activity conferred by N-terminal tyrosines. *, p < 0.05 of BDNF effect compared with vehicle-treated control. #, p < 0.05 for RGS4-expressing oocytes compared with oocytes lacking exogenously expressed RGS4. ζ, p < 0.05 for vehicle-treated oocytes expressing Kir3.4(S143T) compared with Kir3.4(S143T/Y32F/Y53F). B, Kir3.4 GAP-like tyrosine-arginine-glutamine motifs in the N terminus.
