Abstract
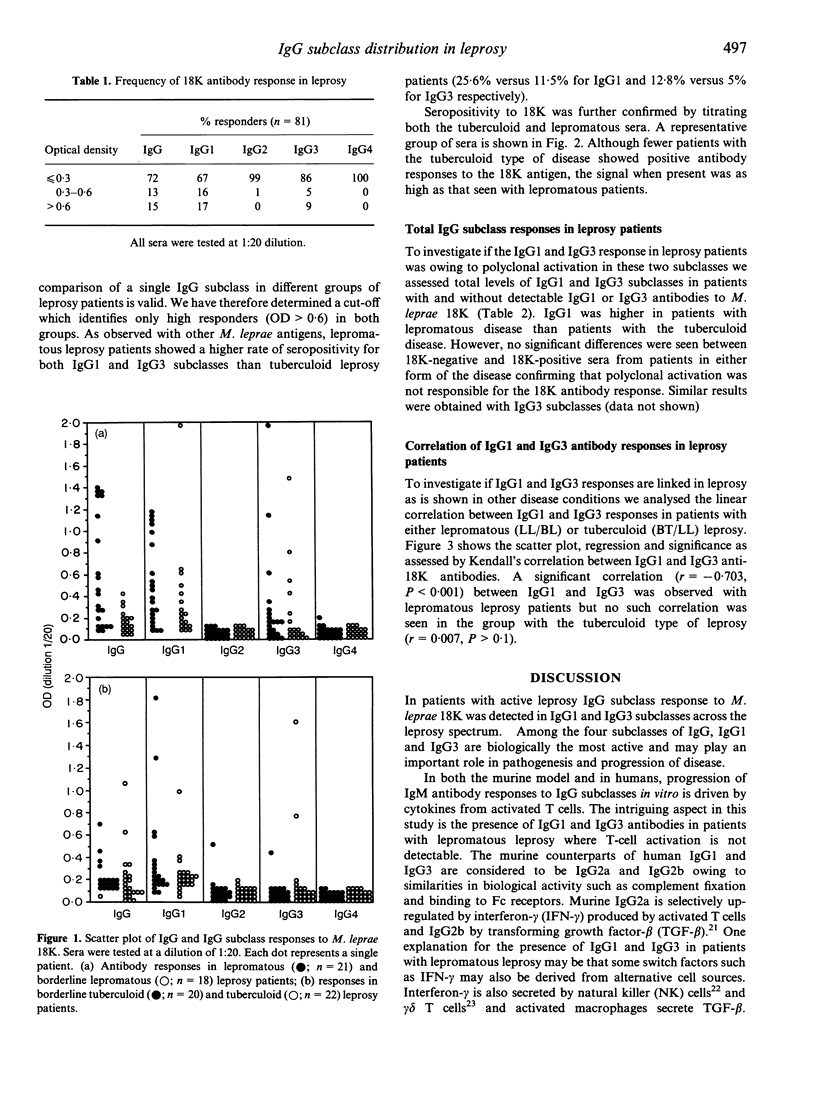
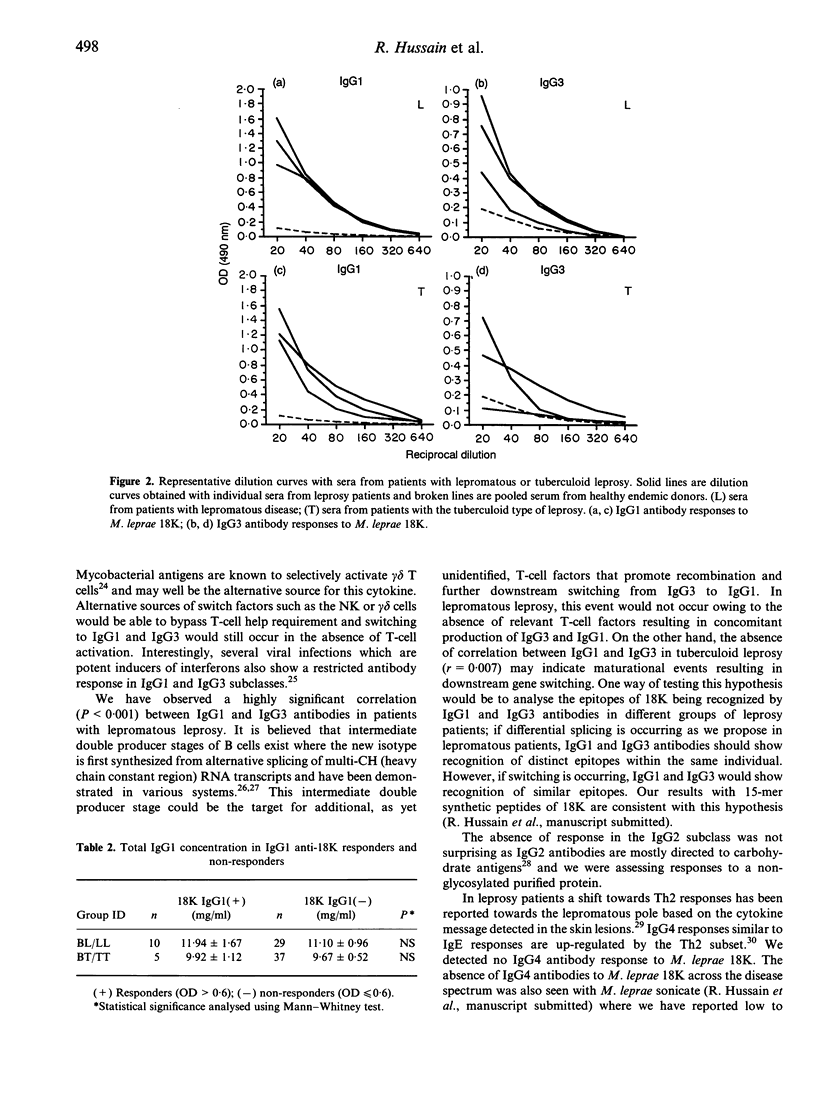
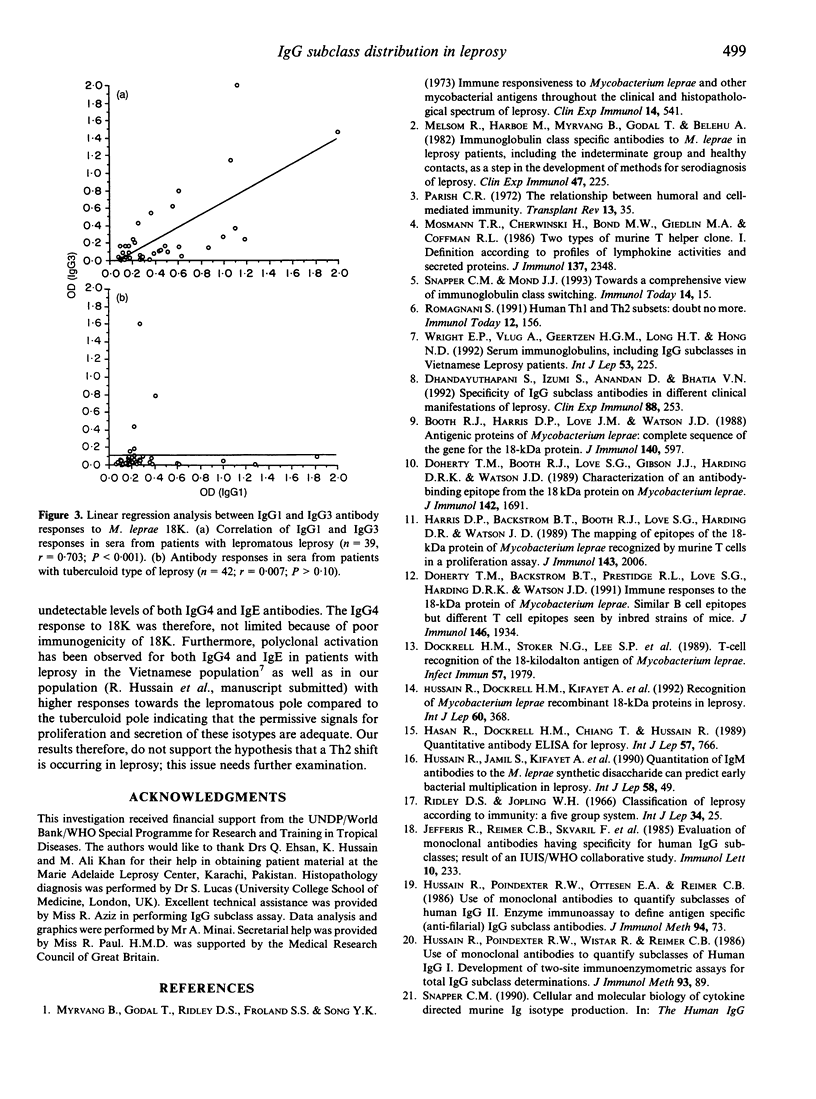
IgG subclass responses to Mycobacterium leprae 18,000 MW recombinant antigen (18K) were determined in sera from untreated leprosy patients using an ELISA-based assay with specific monoclonal antibodies. Antibodies to M. leprae 18K were restricted to IgG1 and IgG3 antibodies with higher seropositivity in lepromatous patients (25.5% for IgG1 and 12.8% for IgG3) compared to patients with tuberculoid disease (11.5% for IgG1 and 5% for IgG3). No significant antibody response was detectable in IgG2 and IgG4 in patients with either lepromatous or tuberculoid leprosy. The selective production of antibodies in IgG1 and IgG3 subclasses could not be related to polyclonal activation in these subclasses as all IgG subclasses showed similar elevated levels at the polyclonal level. The major difference noted between lepromatous and tuberculoid leprosy patients with the IgG subclass antibody response was a strong linear correlation between IgG1 and IgG3 responses to M. leprae 18K in lepromatous patients (r = 0.703, P < 0.001) but not in tuberculoid leprosy patients (r = 0.007, P > 0.10) which may be related to immunoglobulin class switching of IgG3 to IgG1 rather than selective shifts in T-helper subsets. Our results therefore, do not support the hypothesis that activation of Th2 cells occurs in lepromatous leprosy; this issue needs further examination.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth R. J., Harris D. P., Love J. M., Watson J. D. Antigenic proteins of Mycobacterium leprae. Complete sequence of the gene for the 18-kDa protein. J Immunol. 1988 Jan 15;140(2):597–601. [PubMed] [Google Scholar]
- Christmas S. E., Meager A. Production of interferon-gamma and tumour necrosis factor-alpha by human T-cell clones expressing different forms of the gamma delta receptor. Immunology. 1990 Dec;71(4):486–492. [PMC free article] [PubMed] [Google Scholar]
- Dhandayuthapani S., Izumi S., Anandan D., Bhatia V. N. Specificity of IgG subclass antibodies in different clinical manifestations of leprosy. Clin Exp Immunol. 1992 May;88(2):253–257. doi: 10.1111/j.1365-2249.1992.tb03069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell H. M., Stoker N. G., Lee S. P., Jackson M., Grant K. A., Jouy N. F., Lucas S. B., Hasan R., Hussain R., McAdam K. P. T-cell recognition of the 18-kilodalton antigen of Mycobacterium leprae. Infect Immun. 1989 Jul;57(7):1979–1983. doi: 10.1128/iai.57.7.1979-1983.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty T. M., Booth R. J., Love S. G., Gibson J. J., Harding D. R., Watson J. D. Characterization of an antibody-binding epitope from the 18-kDa protein on Mycobacterium leprae. J Immunol. 1989 Mar 1;142(5):1691–1695. [PubMed] [Google Scholar]
- Doherty T. M., Bäckström B. T., Prestidge R. L., Love S. G., Harding D. R., Watson J. D. Immune responses to the 18-kDa protein of Mycobacterium leprae. Similar B cell epitopes but different T cell epitopes seen by inbred strains of mice. J Immunol. 1991 Mar 15;146(6):1934–1940. [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Harris D. P., Bäckström B. T., Booth R. J., Love S. G., Harding D. R., Watson J. D. The mapping of epitopes of the 18-kDa protein of Mycobacterium leprae recognized by murine T cells in a proliferation assay. J Immunol. 1989 Sep 15;143(6):2006–2012. [PubMed] [Google Scholar]
- Hasan R., Dockrell H. M., Chiang T., Hussain R. Quantitative antibody ELISA for leprosy. Int J Lepr Other Mycobact Dis. 1989 Dec;57(4):766–776. [PubMed] [Google Scholar]
- Hussain R., Dockrell H. M., Kifayet A., Daud A., Watson J. D., Chiang T. J., Stoker N. G. Recognition of Mycobacterium leprae recombinant 18-kDa proteins in leprosy. Int J Lepr Other Mycobact Dis. 1992 Sep;60(3):368–375. [PubMed] [Google Scholar]
- Hussain R., Poindexter R. W., Ottesen E. A., Reimer C. B. Use of monoclonal antibodies to quantify subclasses of human IgG. II. Enzyme immunoassay to define antigen specific (anti-filarial) IgG subclass antibodies. J Immunol Methods. 1986 Nov 20;94(1-2):73–80. doi: 10.1016/0022-1759(86)90217-6. [DOI] [PubMed] [Google Scholar]
- Hussain R., Poindexter R. W., Wistar R., Reimer C. B. Use of monoclonal antibodies to quantify subclasses of human IgG. I. Development of two-site immunoenzymometric assays for total IgG subclass determinations. J Immunol Methods. 1986 Oct 23;93(1):89–96. doi: 10.1016/0022-1759(86)90437-0. [DOI] [PubMed] [Google Scholar]
- King C. L., Nutman T. B. Biological role of helper T-cell subsets in helminth infections. Chem Immunol. 1992;54:136–165. [PubMed] [Google Scholar]
- Melsom R., Harboe M., Myrvang B., Godal T., Belehu A. Immunoglobulin class specific antibodies to M. leprae in leprosy patients, including the indeterminate group and healthy contacts as a step in the development of methods for sero-diagnosis of leprosy. Clin Exp Immunol. 1982 Feb;47(2):225–233. [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Myrvang B., Godal T., Ridley D. S., Fröland S. S., Song Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin Exp Immunol. 1973 Aug;14(4):541–553. [PMC free article] [PubMed] [Google Scholar]
- Parish C. R. The relationship between humoral and cell-mediated immunity. Transplant Rev. 1972;13:35–66. doi: 10.1111/j.1600-065x.1972.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Perlmutter A. P., Gilbert W. Antibodies of the secondary response can be expressed without switch recombination in normal mouse B cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7189–7193. doi: 10.1073/pnas.81.22.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skvaril F. IgG subclasses in viral infections. Monogr Allergy. 1986;19:134–143. [PubMed] [Google Scholar]
- Snapper C. M., Mond J. J. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993 Jan;14(1):15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- Wherry J. C., Schreiber R. D., Unanue E. R. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991 May;59(5):1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]


