Abstract
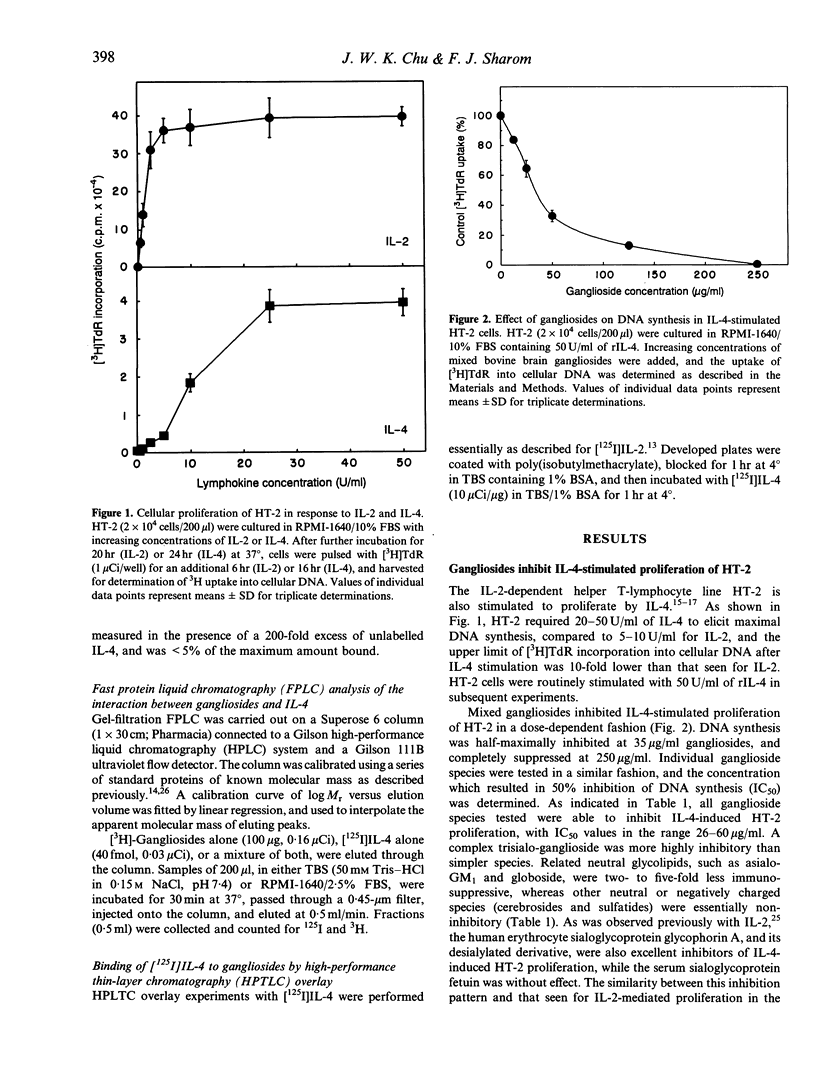
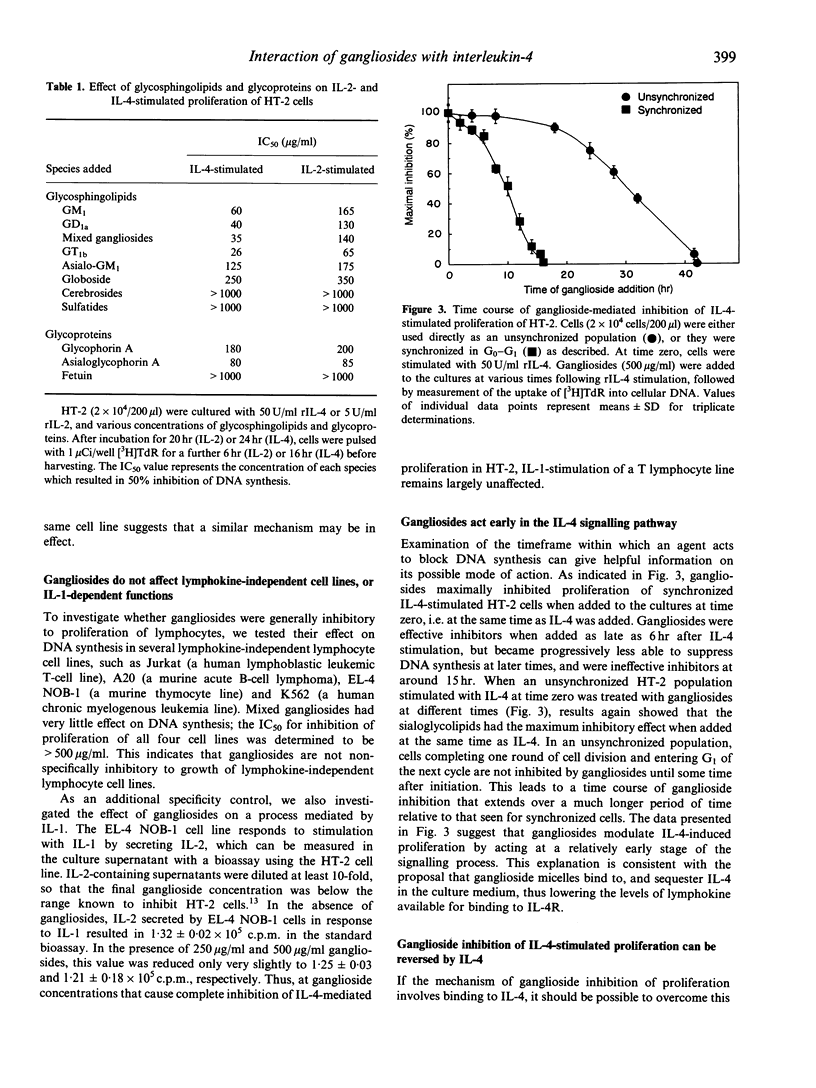
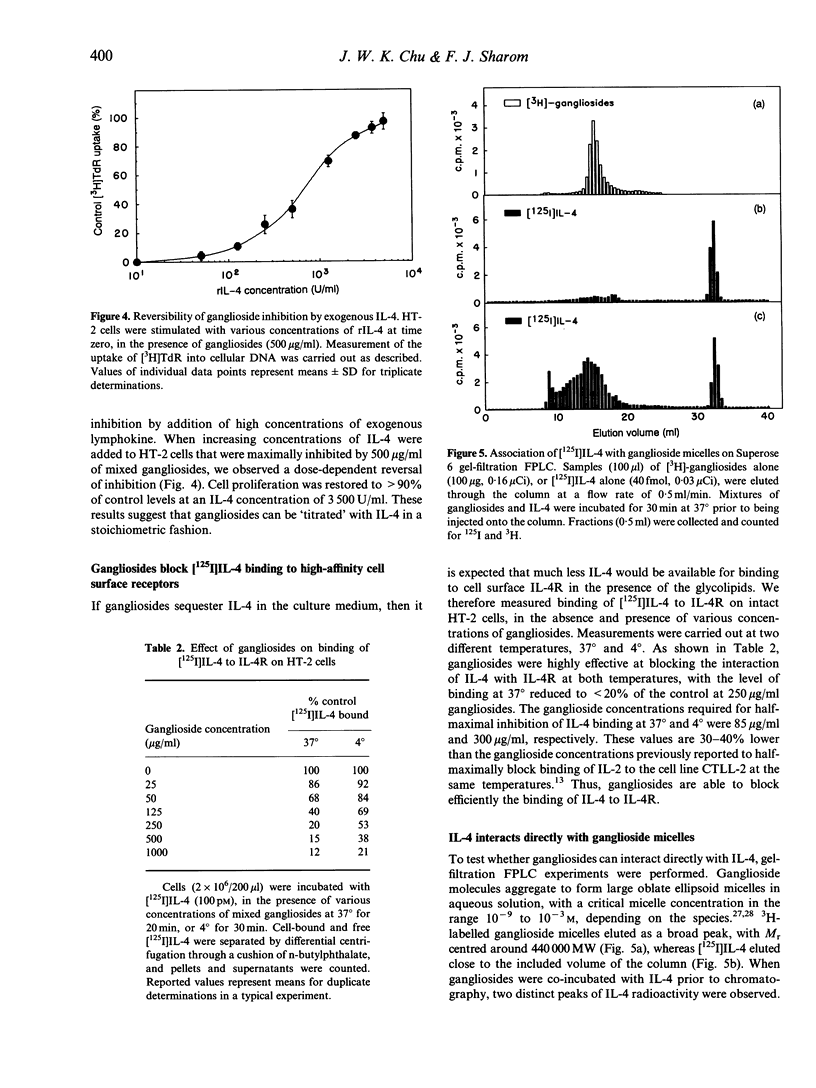
Gangliosides are potent immunosuppressive agents in vitro, and gangliosides shed from tumours in vivo may play an important role in the escape of tumours from immune destruction. We have investigated the effect of gangliosides on interleukin-4 (IL-4)-mediated processes in the murine helper T-cell line HT-2. Various gangliosides inhibited IL-4-stimulated DNA synthesis in HT-2 with IC50 values in the range 26-60 micrograms/ml. However, the proliferation of four lymphokine-independent cell lines was unaffected by 500 micrograms/ml gangliosides, as was the IL-1-stimulated secretion of IL-2 by EL-4 NOB-1 cells. Gangliosides were highly effective inhibitors when added to G0-G1-synchronized HT-2 cells during the first 6 hr after IL-4 stimulation, indicating that they act early in the IL-4 signalling pathway. High levels of exogenous IL-4 completely reversed inhibition of proliferation by gangliosides, which suggests that gangliosides compete with cellular IL-4 receptors for available lymphokine. Receptor-binding experiments confirmed that gangliosides blocked binding of [125I]IL-4 to receptors on intact HT-2 cells in a dose-dependent fashion. Gel-filtration fast protein liquid chromatography (FPLC) demonstrated that [125I]IL-4 co-eluted with ganglioside micelles after co-incubation before chromatography, and an overlay technique showed that IL-4 bound efficiently to gangliosides on thin-layer chromatography plates. Taken together, these results indicate that gangliosides act as potent suppressors of IL-4-dependent processes in lymphocytes, and that their mechanism of action involves direct interaction with IL-4, thus preventing IL-4 binding to high-affinity IL-4 receptors. This information helps to explain the diverse immunosuppressive actions reported for gangliosides, both in vitro and in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine J. A., Schlager J. W., Abraham R. T. Differential effects of interleukin-2 and interleukin-4 on protein tyrosine phosphorylation in factor-dependent murine T cells. Biochim Biophys Acta. 1990 May 2;1052(2):313–322. doi: 10.1016/0167-4889(90)90227-5. [DOI] [PubMed] [Google Scholar]
- Chu J. W., Sharom F. J. Effect of micellar and bilayer gangliosides on proliferation of interleukin-2-dependent lymphocytes. Cell Immunol. 1991 Feb;132(2):319–338. doi: 10.1016/0008-8749(91)90031-6. [DOI] [PubMed] [Google Scholar]
- Chu J. W., Sharom F. J. Gangliosides inhibit T-lymphocyte proliferation by preventing the interaction of interleukin-2 with its cell surface receptors. Immunology. 1993 May;79(1):10–17. [PMC free article] [PubMed] [Google Scholar]
- Chu J. W., Sharom F. J. Glycophorin A interacts with interleukin-2 and inhibits interleukin-2-dependent T-lymphocyte proliferation. Cell Immunol. 1992 Dec;145(2):223–239. doi: 10.1016/0008-8749(92)90327-l. [DOI] [PubMed] [Google Scholar]
- Chu J. W., Sharom F. J. Interleukin-2 binds to gangliosides in micelles and lipid bilayers. Biochim Biophys Acta. 1990 Oct 19;1028(3):205–214. doi: 10.1016/0005-2736(90)90168-n. [DOI] [PubMed] [Google Scholar]
- Corti M., Degiorgio V., Ghidoni R., Sonnino S., Tettamanti G. Laser-light scattering investigation of the micellar properties of gangliosides. Chem Phys Lipids. 1980 Apr;26(3):225–238. doi: 10.1016/0009-3084(80)90053-5. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Fasman G. D., Lodish H. F. A new hematopoietic growth factor receptor superfamily: structural features and implications for signal transduction. Curr Opin Cell Biol. 1990 Aug;2(4):648–651. doi: 10.1016/0955-0674(90)90106-o. [DOI] [PubMed] [Google Scholar]
- Dyatlovitskaya E. V., Bergelson L. D. Glycosphingolipids and antitumor immunity. Biochim Biophys Acta. 1987 Jul 8;907(2):125–143. doi: 10.1016/0304-419x(87)90002-3. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R., Krammer P. H., Diamantstein T., Uhr J. W., Vitetta E. S. B cell-stimulatory factor 1 (BSF-1) promotes growth of helper T cell lines. J Exp Med. 1986 Aug 1;164(2):580–593. doi: 10.1084/jem.164.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett D. S., Powers R., March C. J., Frieden E. A., Clore G. M., Gronenborn A. M. Determination of the secondary structure and folding topology of human interleukin-4 using three-dimensional heteronuclear magnetic resonance spectroscopy. Biochemistry. 1992 May 5;31(17):4347–4353. doi: 10.1021/bi00132a027. [DOI] [PubMed] [Google Scholar]
- Hoon D. S., Irie R. F., Cochran A. J. Gangliosides from human melanoma immunomodulate response of T cells to interleukin-2. Cell Immunol. 1988 Feb;111(2):410–419. doi: 10.1016/0008-8749(88)90104-9. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Nakakuma H., Shionoya H., Kawaguchi T., Yamatsu K., Takatsuki K. Ganglioside-induced inhibition of in vivo immune response in mice. Life Sci. 1992;51(11):847–851. doi: 10.1016/0024-3205(92)90612-s. [DOI] [PubMed] [Google Scholar]
- Ladisch S., Kitada S., Hays E. F. Gangliosides shed by tumor cells enhance tumor formation in mice. J Clin Invest. 1987 Jun;79(6):1879–1882. doi: 10.1172/JCI113031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. M., Grant C. W. Headgroup oligosaccharide dynamics of a transmembrane glycoprotein. Can J Biochem. 1980 Oct;58(10):1197–1205. doi: 10.1139/o80-160. [DOI] [PubMed] [Google Scholar]
- Loe D. W., Glover J. R., Head S., Sharom F. J. Solubilization, characterization, and detergent interactions of lymphocyte 5'-nucleotidase. Biochem Cell Biol. 1989 Apr-May;67(4-5):214–223. doi: 10.1139/o89-033. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Castle B. E., Christiansen J., Schreurs J., Rennick D., Arai N., Hoy P., Takebe Y., Howard M. Expression of high affinity receptors for murine interleukin 4 (BSF-1) on hemopoietic and nonhemopoietic cells. J Immunol. 1988 Jan 15;140(2):456–464. [PubMed] [Google Scholar]
- Marcus D. M. A review of the immunogenic and immuno-modulatory properties of glycosphingolipids. Mol Immunol. 1984 Nov;21(11):1083–1091. doi: 10.1016/0161-5890(84)90118-4. [DOI] [PubMed] [Google Scholar]
- Minami Y., Kono T., Miyazaki T., Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- Prokazova N. V., Dyatlovitskaya E. V., Bergelson L. D. Sialylated lactosylceramides. Possible inducers of non-specific immunosuppression and atherosclerotic lesions. Eur J Biochem. 1988 Feb 15;172(1):1–6. doi: 10.1111/j.1432-1033.1988.tb13847.x. [DOI] [PubMed] [Google Scholar]
- Redfield C., Smith L. J., Boyd J., Lawrence G. M., Edwards R. G., Smith R. A., Dobson C. M. Secondary structure and topology of human interleukin 4 in solution. Biochemistry. 1991 Nov 19;30(46):11029–11035. doi: 10.1021/bi00110a004. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Wilkinson T. M., Sheng L. Isolation of glycophorin with deoxycholate. Biochim Biophys Acta. 1979 Jul 5;554(2):533–537. doi: 10.1016/0005-2736(79)90389-4. [DOI] [PubMed] [Google Scholar]
- Sekaly R. P., MacDonald H. R., Nabholz M., Smith K. A., Cerottini J. C. Regulation of the rate of cell cycle progression in quiescent cytolytic T cells by T cell growth factor: analysis by flow microfluorometry. J Cell Physiol. 1984 Oct;121(1):159–166. doi: 10.1002/jcp.1041210120. [DOI] [PubMed] [Google Scholar]
- Shan X., Davis J. H., Chu J. W., Sharom F. J. 2H-NMR investigation of DMPC/glycophorin bilayers. Biochim Biophys Acta. 1994 Jul 13;1193(1):127–137. doi: 10.1016/0005-2736(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Sharom F. J., Chiu A. L., Chu J. W. Membrane gangliosides modulate interleukin-2-stimulated T-lymphocyte proliferation. Biochim Biophys Acta. 1991 Aug 13;1094(1):35–42. doi: 10.1016/0167-4889(91)90023-q. [DOI] [PubMed] [Google Scholar]
- Sharom F. J., Chiu A. L., Ross T. E. Gangliosides and glycophorin inhibit T-lymphocyte activation. Biochem Cell Biol. 1990 Apr;68(4):735–744. doi: 10.1139/o90-106. [DOI] [PubMed] [Google Scholar]
- Sherblom A. P., Sathyamoorthy N., Decker J. M., Muchmore A. V. IL-2, a lectin with specificity for high mannose glycopeptides. J Immunol. 1989 Aug 1;143(3):939–944. [PubMed] [Google Scholar]
- Spiegel S., Fishman P. H., Weber R. J. Direct evidence that endogenous GM1 ganglioside can mediate thymocyte proliferation. Science. 1985 Dec 13;230(4731):1285–1287. doi: 10.1126/science.2999979. [DOI] [PubMed] [Google Scholar]
- Ulrich-Bott B., Wiegandt H. Micellar properties of glycosphingolipids in aqueous media. J Lipid Res. 1984 Nov;25(11):1233–1245. [PubMed] [Google Scholar]
- Veh R., Corfield A. P., Sander M., Schauer R. Neuraminic acid-specific modification and tritium labelling of gangliosides. Biochim Biophys Acta. 1976 Jan 18;486(1):145–160. doi: 10.1016/0005-2760(77)90079-0. [DOI] [PubMed] [Google Scholar]



