Abstract
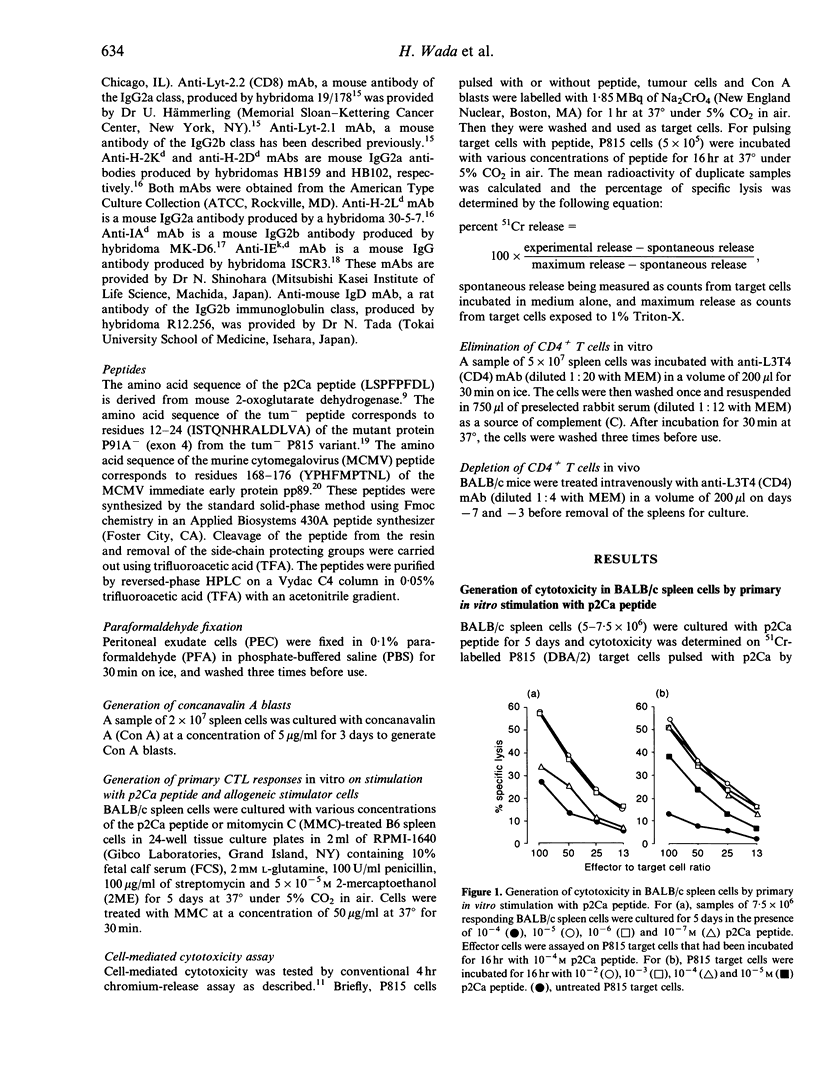
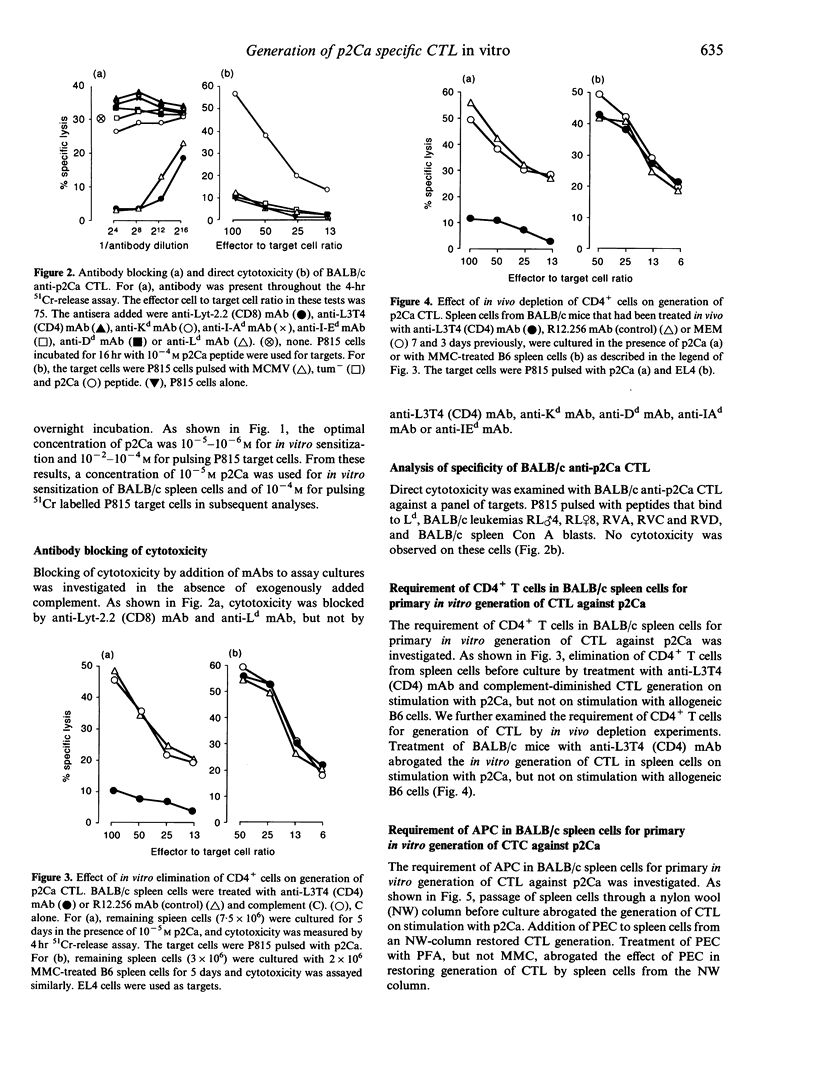
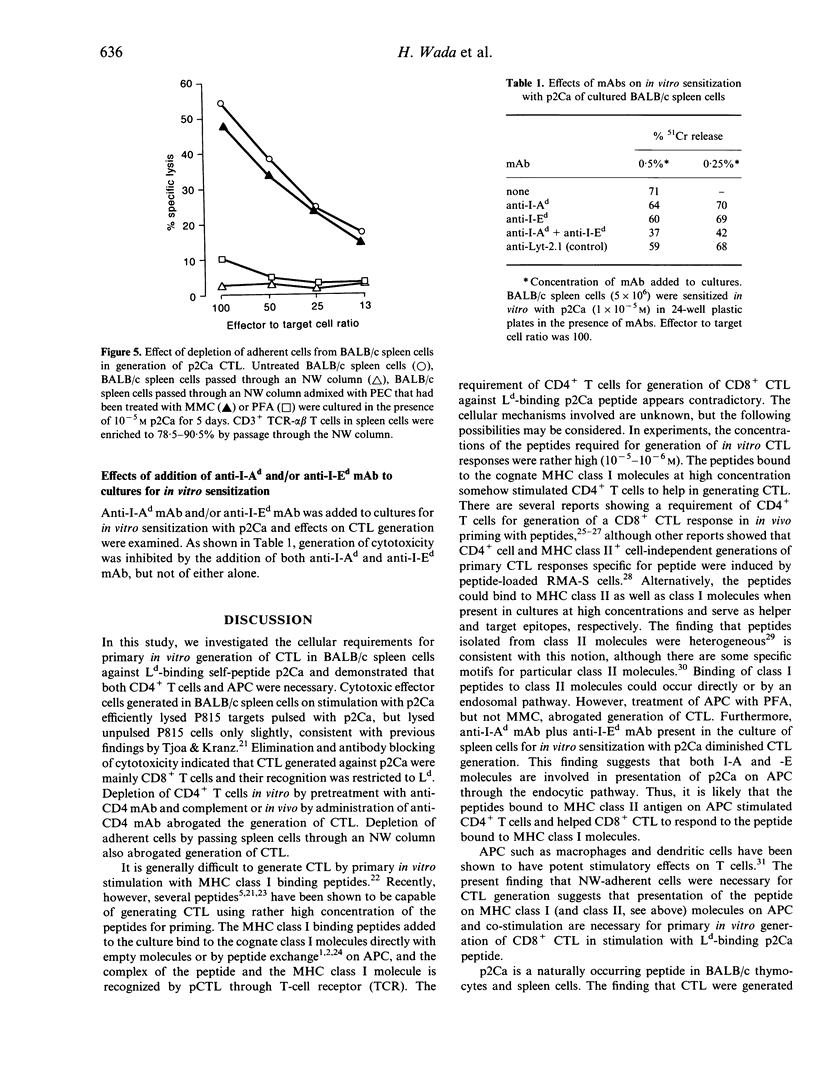
We investigated the cellular requirement for primary in vitro generation of cytotoxic T-lymphocytes (CTL) in BALB/c spleen cells against Ld-binding self-peptide p2Ca. Depletion of CD4+ T-cells in vitro by pretreatment with anti-CD4 monoclonal antibody (mAb) and complement or in vivo by administration of anti-CD4 mAb abrogated generation of CTL. Depletion of adherent cells by passing spleen cells through a nylon wool (NW) column also abrogated generation of CTL. Addition of peritoneal exudate cells (PEC) to spleen cells passed through the NW column restored CTL generation. These findings indicate that both CD4+ T-cells and antigen-presenting cells (APC) were necessary for CTL generation. Treatment of PEC with paraformaldehyde (PFA), but not mitomycin-C (MMC) abrogated their ability to restore CTL generation when mixed with spleen cells from the NW column, suggesting that an endocytic pathway could be involved in presentation of p2Ca on APC.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. A., Damico C. A., Wieties K. M., Hansen T. H., Connolly J. M. Correlation between CD8 dependency and determinant density using peptide-induced, Ld-restricted cytotoxic T lymphocytes. J Exp Med. 1991 Apr 1;173(4):849–858. doi: 10.1084/jem.173.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone F. R., Bevan M. J. Induction of ovalbumin-specific cytotoxic T cells by in vivo peptide immunization. J Exp Med. 1989 Mar 1;169(3):603–612. doi: 10.1084/jem.169.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone F. R., Moore M. W., Sheil J. M., Bevan M. J. Induction of cytotoxic T lymphocytes by primary in vitro stimulation with peptides. J Exp Med. 1988 Jun 1;167(6):1767–1779. doi: 10.1084/jem.167.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis E., Tsai V., Crimi C., DeMars R., Wentworth P. A., Chesnut R. W., Grey H. M., Sette A., Serra H. M. Induction of anti-tumor cytotoxic T lymphocytes in normal humans using primary cultures and synthetic peptide epitopes. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2105–2109. doi: 10.1073/pnas.91.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruijn M. L., Schumacher T. N., Nieland J. D., Ploegh H. L., Kast W. M., Melief C. J. Peptide loading of empty major histocompatibility complex molecules on RMA-S cells allows the induction of primary cytotoxic T lymphocyte responses. Eur J Immunol. 1991 Dec;21(12):2963–2970. doi: 10.1002/eji.1830211210. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Fayolle C., Deriaud E., Leclerc C. In vivo induction of cytotoxic T cell response by a free synthetic peptide requires CD4+ T cell help. J Immunol. 1991 Dec 15;147(12):4069–4073. [PubMed] [Google Scholar]
- GORER P. A. Studies in antibody response of mice to tumour inoculation. Br J Cancer. 1950 Dec;4(4):372–379. doi: 10.1038/bjc.1950.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos F. J., Müllbacher A. Induction of primary anti-viral cytotoxic T cells by in vitro stimulation with short synthetic peptide and interleukin-7. Eur J Immunol. 1992 Dec;22(12):3183–3185. doi: 10.1002/eji.1830221224. [DOI] [PubMed] [Google Scholar]
- Kranz D. M., Sherman D. H., Sitkovsky M. V., Pasternack M. S., Eisen H. N. Immunoprecipitation of cell surface structures of cloned cytotoxic T lymphocytes by clone-specific antisera. Proc Natl Acad Sci U S A. 1984 Jan;81(2):573–577. doi: 10.1073/pnas.81.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasarte J. J., Sarobe P., Gullón A., Prieto J., Borrás-Cuesta F. Induction of cytotoxic T lymphocytes in mice against the principal neutralizing domain of HIV-1 by immunization with an engineered T-cytotoxic-T-helper synthetic peptide construct. Cell Immunol. 1992 Apr 15;141(1):211–218. doi: 10.1016/0008-8749(92)90140-k. [DOI] [PubMed] [Google Scholar]
- Lurquin C., Van Pel A., Mariamé B., De Plaen E., Szikora J. P., Janssens C., Reddehase M. J., Lejeune J., Boon T. Structure of the gene of tum- transplantation antigen P91A: the mutated exon encodes a peptide recognized with Ld by cytolytic T cells. Cell. 1989 Jul 28;58(2):293–303. doi: 10.1016/0092-8674(89)90844-1. [DOI] [PubMed] [Google Scholar]
- Maryanski J. L., Pala P., Corradin G., Jordan B. R., Cerottini J. C. H-2-restricted cytolytic T cells specific for HLA can recognize a synthetic HLA peptide. Nature. 1986 Dec 11;324(6097):578–579. doi: 10.1038/324578a0. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Rothbard J. HLA B37 determines an influenza A virus nucleoprotein epitope recognized by cytotoxic T lymphocytes. J Exp Med. 1986 Nov 1;164(5):1397–1406. doi: 10.1084/jem.164.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama E. Blocking of effector cell cytotoxicity and T-cell proliferation by Lyt antisera. Immunol Rev. 1982;68:117–134. doi: 10.1111/j.1600-065x.1982.tb01062.x. [DOI] [PubMed] [Google Scholar]
- Nakayama E., Shiku H., Takahashi T., Oettgen H. F., Old L. J. Definition of a unique cell surface antigen of mouse leukemia RL male 1 by cell-mediated cytotoxicity. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3486–3490. doi: 10.1073/pnas.76.7.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Hansen T. H., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. II. Antibodies to the H-2Ld antigen, the products of a third polymorphic locus of the mouse major histocompatibility complex. J Immunol. 1980 Dec;125(6):2473–2477. [PubMed] [Google Scholar]
- Rammensee H. G., Falk K., Rötzschke O. MHC molecules as peptide receptors. Curr Opin Immunol. 1993 Feb;5(1):35–44. doi: 10.1016/0952-7915(93)90078-7. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Rothbard J. B., Koszinowski U. H. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature. 1989 Feb 16;337(6208):651–653. doi: 10.1038/337651a0. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Rothstein L., Gamble S., Fleischacker C. Characterization of antigen-presenting cells that present exogenous antigens in association with class I MHC molecules. J Immunol. 1993 Jan 15;150(2):438–446. [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., al-Ramadi B. K., Rothbard J., Janeway C. A., Jr Truncation variants of peptides isolated from MHC class II molecules suggest sequence motifs. Nature. 1992 Oct 1;359(6394):429–431. doi: 10.1038/359429a0. [DOI] [PubMed] [Google Scholar]
- Schild H., Rötzschke O., Kalbacher H., Rammensee H. G. Limit of T cell tolerance to self proteins by peptide presentation. Science. 1990 Mar 30;247(4950):1587–1589. doi: 10.1126/science.2321019. [DOI] [PubMed] [Google Scholar]
- Stockert E., DeLeo A. B., O'Donnell P. V., Obata Y., Old L. J. G(AKSL2): a new cell surface antigen of the mouse related to the dualtropic mink cell focus-inducing class of murine leukemia virus detected by naturally occurring antibody. J Exp Med. 1979 Jan 1;149(1):200–215. doi: 10.1084/jem.149.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjoa B. A., Kranz D. M. Generation of cytotoxic T-lymphocytes to a self-peptide/class I complex: a model for peptide-mediated tumor rejection. Cancer Res. 1994 Jan 1;54(1):204–208. [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Udaka K., Tsomides T. J., Eisen H. N. A naturally occurring peptide recognized by alloreactive CD8+ cytotoxic T lymphocytes in association with a class I MHC protein. Cell. 1992 Jun 12;69(6):989–998. doi: 10.1016/0092-8674(92)90617-l. [DOI] [PubMed] [Google Scholar]
- Udaka K., Tsomides T. J., Walden P., Fukusen N., Eisen H. N. A ubiquitous protein is the source of naturally occurring peptides that are recognized by a CD8+ T-cell clone. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11272–11276. doi: 10.1073/pnas.90.23.11272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttenhove C., Van Snick J., Boon T. Immunogenic variants obtained by mutagenesis of mouse mastocytoma P815. I. Rejection by syngeneic mice. J Exp Med. 1980 Nov 1;152(5):1175–1183. doi: 10.1084/jem.152.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Suzuki T., Taniguchi M., Shinohara N. Monoclonal anti-Ia murine alloantibodies crossreactive with the Ia-homologues of other mammalian species including humans. Transplantation. 1983 Dec;36(6):712–718. doi: 10.1097/00007890-198336060-00025. [DOI] [PubMed] [Google Scholar]


