FIGURE 2.
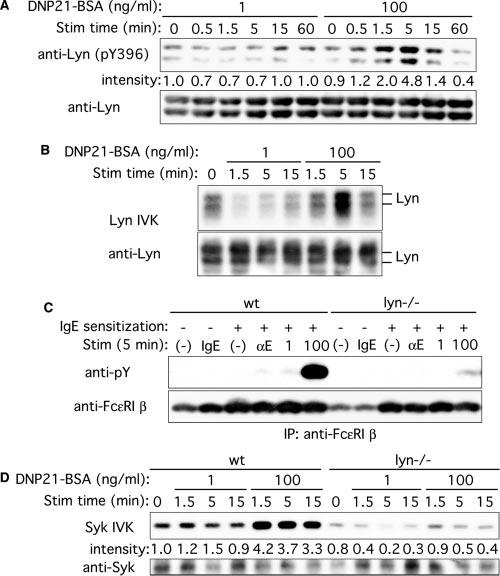
Lyn kinase activity, tyrosine phosphorylation of Fc∊RI β, and Syk kinase activity upon stimulation with low and high concentrations of Ag. A and B, Wt BMMC were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA for the indicated periods. Cell lysates were directly analyzed by immunoblotting with anti-phospho-Src (Tyr416) that interacts with Tyr396-phosphorylated Lyn, followed by reprobing the same blot with anti-Lyn Ab (A). Lyn was immunoprecipitated from lysates and submitted to in vitro kinase (IVK) assays (B). The same blot was probed with anti-Lyn Ab. C, wt and lyn−/− BMMCs were incubated without or with 5 μg/ml SPE-7 IgE (IgE). Alternatively, these cells were IgE sensitized and stimulated with 2 μg/ml anti-IgE (αE) or 1 or 100 ng/ml DNP21-BSA for 5 min. Fc∊RI β subunit was immunoprecipitated and submitted to immunoblotting with anti-phosphotyrosine mAb, followed by reprobing with anti-Fc∊RI β mAb. D, Wt and lyn−/− BMMCs were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA for the indicated periods. Syk was immunoprecipitated from lysates and submitted to in vitro kinase assays. The same blot was probed with anti-Syk Ab. Relative band intensities of p56lyn phosphorylation (A) and Syk (D) normalized against their amounts, as determined by densitometry, are shown. The results shown were reproduced in an additional experiment using an independent set of BMMCs.
