FIGURE 6.
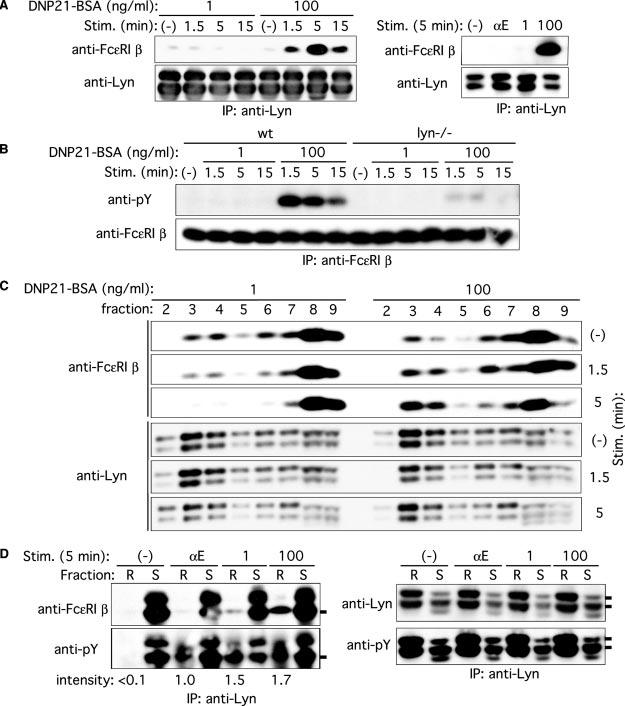
Decreased and increased Fc∊RI β/Lyn association and Fc∊RI β localization to lipid rafts upon stimulation with low and high concentrations of Ag, respectively. A, wt BMMC were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA or 2 μg/ml anti-IgE mAb B1E3 (αE) for the indicated periods. Cell lysates were immunoprecipitated with anti-Lyn, and Lyn immune complexes were subjected to immunoblotting with anti-Fc∊RI β mAb. The same blots were reprobed with anti-Lyn Ab. Results representative of two experiments are shown. B, wt and lyn−/− BMMC were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA for the indicated periods. Fc∊RI β was immunoprecipitated and analyzed by immunoblotting with anti-phosphotyrosine mAb, followed by reprobing with anti-Fc∊RI β. C, wt BMMC were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA for the indicated periods. Cell lysates were fractionated by ultracentrifugation through a sucrose density gradient. Fractions were analyzed by immunoblotting with anti-Fc∊RI β mAb or anti-Lyn. Fractions 2–4 contain lipid rafts, and fractions 6–9 contain soluble proteins. The reduced and increased raft localizations of Fc∊RI β depending on Ag concentration were reproduced in two additional experiments. D, wt BMMC were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA or 2 μg/ml anti-IgE mAb B1E3 (αE) for 5 min. Cell lysates were fractioned by ultracentrifugation, and raft (R) and soluble fractions (S) were pooled and immunoprecipitated with anti-Lyn. Lyn immune complexes were analyzed by immunoblotting with anti-Fc∊RI β, anti-phosphotyrosine, or anti-Lyn mAb. Relative band intensities of Fc∊RI β phosphorylation in lipid rafts are shown. Phosphorylation induced with anti-IgE is set at 1.0.
