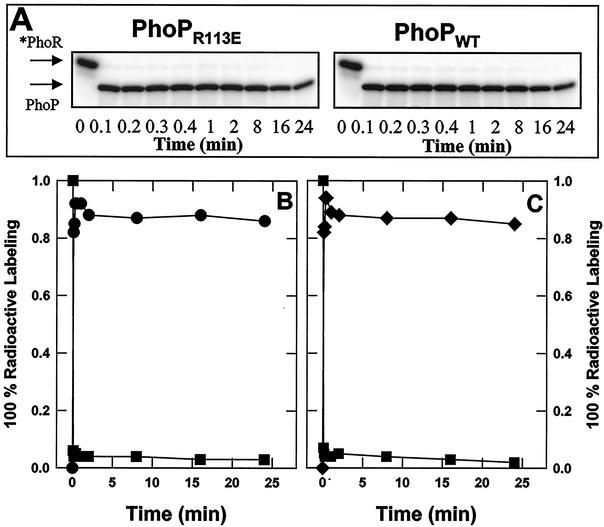
FIG. 6.
Phosphotransfer rates from *PhoR∼P to PhoPR113E and PhoPWT are similar. GST-*PhoR was phosphorylated by incubating it with [γ-32P]ATP and was bound to glutathione-agarose. After excess [γ-32P]ATP was removed, 10 U of thrombin was added to the beads and mixed at room temperature for 20 min. The PhoR∼P was separated from the beads and mixed with an equimolar amount of PhoPR113E or PhoPWT. Samples having the same volume were removed from each reaction mixture at different times, as indicated, and the reaction was stopped with SDS loading buffer. Samples were then subjected to SDS-PAGE. The gels were dried, and the radioactivity was quantified with a PhosphorImager. Phosphorylated protein contents were expressed in arbitrary units. (A) Radioactivity in SDS-PAGE profile. The migration positions of *PhoR∼P and PhoP∼P are indicated by arrows. (B) *PhoR∼P incubated with PhoPR113E. (C) *PhoR∼P incubated with PhoPWT. Symbols: ▪, *PhoR∼P; •, PhoPR113E∼P; ⧫, PhoPWT∼P.