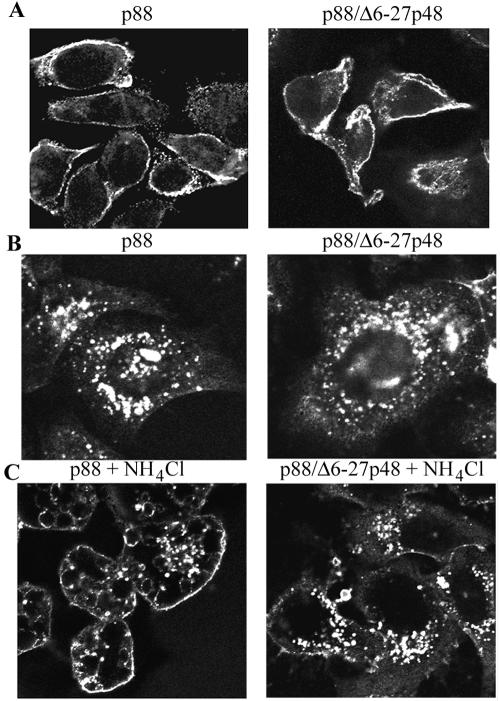
FIG. 5.
Effects of Δ6-27p48 VacA dominant-negative protein on binding and internalization of wild-type VacA. (A) HeLa cells were intoxicated for 1 h at 37°C with wild-type acid-activated VacA (p88) purified from an H. pylori culture supernatant (left panel) or with a mixture of wild-type VacA (p88) and Δ6-27p48 (right panel). The capacity of the VacA proteins to interact with the cells was assessed by indirect immunofluorescence, using an anti-VacA polyclonal antiserum reactive with the C-terminal region of p88 VacA, as described in Materials and Methods. (B and C) Wild-type acid-activated VacA (p88) purified from an H. pylori culture supernatant (left panels) or a mixture of p88 and Δ6-27p48 (right panels) was added to HeLa cells in the absence (B) or presence (C) of 10 mM ammonium chloride, and cells were then incubated for 12 h at 37°C. The ability of wild-type VacA (p88) to enter cells was assessed by indirect immunofluorescence of permeabilized cells, using anti-VacA polyclonal antiserum.