Figure 4.
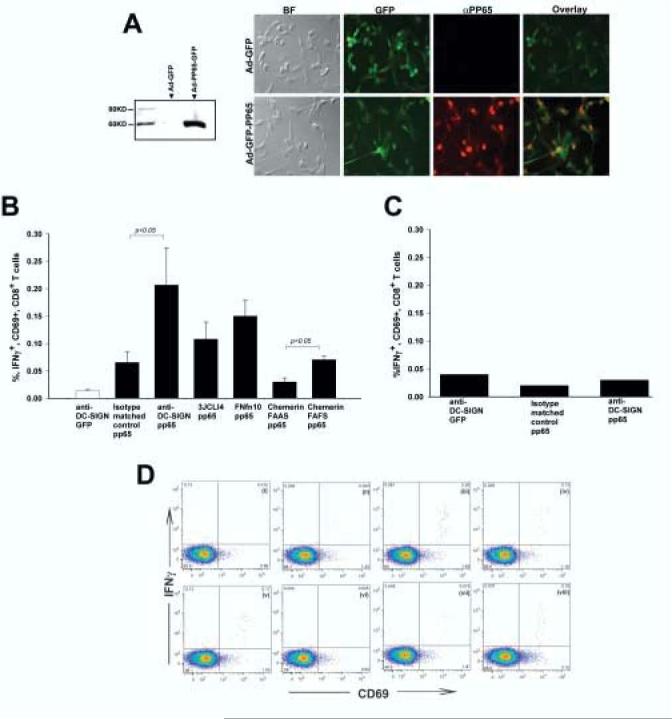
DC transduced with targeted derivatives of Ad-FBAP-pp65 are more efficient at stimulating antigen-specific autologous CD8+ T cells. (A) Efficient expression of HCMV pp65 by Ad-FBAP-pp65. (Left panel) To confirm expression of the pp65 expression cassette, HeLa cells were transduced with Ad-FBAP-pp65. Forty-eight hours thereafter cells were lysed and an immunoblot was performed on nuclear extracts with an anti-pp65 antibody. No immunoreactive protein was detected in lysate from Ad-FBAP-GFP transduced cells (lane 1), while a band of the expected 65 kDa MW was detected in lysate from cells transduced with Ad-FBAP-pp65 vector (lane 2) (Right panel) To confirm expression of pp65 in primary human DC, Ad-FBAP-pp65 or Ad-FBAP-GFP was used to infect cells and twenty-four hours later an immunofluorescence assay was performed using Alexa Fluor 594 conjugated secondary antibodies for anti-pp65. Virally-encoded GFP was also visualized (since both constructs encode GFP); overlay images for GFP and pp65 expression confirmed that nuclear pp65 was detected specifically in cells that also coexpressed GFP (as expected). BF denotes a microscopic image that was recorded under bright field illumination; final magnification in these panels is 200x. (B) Targeting of Ad-FBAP-pp65 to DC-SIGN, ChemR23 or αvβ3 integrin results in a functional increase in specific antigen presentation by vector-transduced human DC. Monocyte-derived human DC from a CMV seropositive donor were transduced with (i) anti-DC-SIGN conjugated Ad-FBAP-GFP virus (“anti-DC-SIGN GFP”), (ii) Ad-FBAP-pp65 virus that was conjugated to an irrelevant monoclonal antibody of the same isotype (“isotype matched control pp65”), (iii) anti-DC-SIGN conjugated Ad-FBAP-pp65 virus (“anti-DC-SIGN pp65”), (iv) a high- affinity and highly selective αvβ3 integrin-binding peptide (3JCLI4), (v) a low-affinity and relatively non-selective αvβ3 integrin-binding (FnFn10), (vi) a mutated derivative of a ChemR23-binding peptide, which contains an alanine substitution in a key residue (chemerin FAAS), and (vii) the wild-type version of this same peptide, which binds to ChemR23 with nanomolar affinity (chemerin FAFS). 48h following vector transduction, the DC were mixed with autologous PBMC. After a further 18h, cells were harvested and analyzed for expression of intracellular IFNγ (as well as cell surface expression of CD3, CD8 and CD69). The results shown represent mean values from three independent experiments; bars denote the standard error of the mean. Statistically significant differences are indicated with p values over the compared groups (t test). (C) Interferon-gamma responses are pp65-specific. Monocyte-derived DC from a CMV seronegative donor were transduced with (i) an anti-DCSIGN antibody conjugated to Ad-FBAP-GFP, (ii) Ad-FBAP-pp65 conjugated to an isotype matched control antibody or (iii) Ad-FBAP-pp65 conjugated to an anti-DC-SIGN antibody. The experiment was performed in the same manner as in 4B. Note that in the CMV seronegative donor, no specific enhancement of intracellular IFNγ production was observed with Ad-FBAP encoding pp65 vs. GFP, underlining the antigen specificity of the assay performed in 4B. (D) Representative intracellular cytokine staining results, corresponding to the data summarized in (B). CD69 expression is plotted on the X-axis, and intracellular IFN-γ expression on the Y-axis; all cells shown in this plot were gated to be CD3+ and CD8+. IFN-γ detection is shown from PBMC incubated with autologous, monocyte-derived DC from a CMV-seropositive donor that were transduced with (i) anti-DC-SIGN conjugated Ad-FBAP-GFP virus (negative control), or Ad-FBAP-pp65 virus that was conjugated to the following ligands: (ii) an irrelevant monoclonal antibody of the same isotype (“isotype matched control pp65”), (iii) an anti-DC-SIGN antibody (“anti-DC-SIGN pp65”), (iv) a high- affinity and highly selective αvβ3 integrin-binding peptide (3JCLI4), (v) a low-affinity and relatively non-selective αvβ3 integrin-binding (FnFn10), (vi) a mutated derivative of a ChemR23-binding peptide, which contains an alanine substitution in a key residue (chemerin FAAS), and (vii) the wild-type version of this same peptide, which binds to ChemR23 with nanomolar affinity (chemerin FAFS). (viii) displays data from PBMC that were incubated with autologous DC that had been pulsed with a pool of overlapping peptides spanning CMV pp65. Numbers in each panel denote the percentage of cells within each of the defined quadrants in the flow cytometric analysis.
