Abstract
Objective
To evaluate prospectively long-term quality of life and functional outcome after restorative proctocolectomy (RPC) with ileal pouch-anal anastomosis, and to evaluate and validate a novel quality-of-life indicator in this group of patients.
Summary Background Data
Restorative proctocolectomy with ileal pouch-anal anastomosis is now the preferred option when total proctocolectomy is required for ulcerative colitis or familial adenomatous polyposis, but long-term data on functional outcome and quality of life after the procedure are lacking.
Methods
Patients (n = 977) who underwent RPC with stapled anastomosis for colitis or polyposis coli and who were followed for ≥12 months were included. Quality of life, fecal incontinence, and satisfaction with surgery were prospectively evaluated by structured interview or questionnaire for 1 to 12 years after surgery (median 5.0). Quality of life was scored using the Cleveland Global Quality of Life (CGQL) instrument (Fazio Score). This is a novel score developed over the past 15 years by the senior author. Quality of life was also evaluated in a subgroup of patients with the Short Form 36 (SF-36). The CGQL was validated by determining its reliability, responsiveness, and validity as well as its correlation with the SF-36 score.
Results
Postoperative quality of life as measured by SF-36 was excellent and compared well with published norms for the general U.S. population. The CGQL was found to be reliable, responsive, and valid, and there was a high correlation with the SF-36 scores. Using the CGQL, quality of life was shown to increase after the first 2 years after surgery, and there was no deterioration thereafter. The prevalence of perfect continence increased from 75.5% before surgery to 82.4% after surgery, and although this deteriorated somewhat >2 years after surgery, it was no worse than preoperative values. Ninety-eight percent of patients would recommend the surgery to others.
Conclusions
Long-term quality of life after ileal pouch surgery is excellent and the level of continence is satisfactory. This surgery is an excellent long-term option in patients requiring total proctocolectomy. The CGQL is a simple, valid, and reliable measure of quality of life after pelvic pouch surgery and may well be applicable in many other clinical conditions.
Reconstructive proctocolectomy (RPC) with formation of ileal pouch-anal anastomosis (IPAA) was introduced by Parks and Nicholls in 1978. 1 Panproctocolectomy with IPAA is considered the preferred option for the surgical treatment of inflammatory bowel disease because it removes the diseased bowel, reduces the risk of cancer, and preserves a natural route for defecation while maintaining fecal continence and avoiding the need for a permanent stoma. 2 Restorative proctocolectomy is also recommended for the majority of patients with familial adenomatous polyposis, particularly where the rectum is significantly affected by adenomas. We and others have shown that the operation is safe and effective in the majority of patients. 3–5
With the complication rates consistently low, the emphasis is increasingly on the assessment of quality of life and function in these patients. However, there are few available data on long-term quality of life after pouch surgery using validated instruments. Although there are a number of validated generic 6,7 or inflammatory bowel disease-specific 8–10 quality-of-life instruments that may be of use in the evaluation of these patients, the large numbers of questions and the time and labor involved in their administration make application to large numbers of patients on a serial basis difficult.
A valid, short quality-of-life instrument that could be self-administered would be useful for the serial evaluation of quality of life after IPAA. However, at the beginning of this study in 1986, no such instrument was available. We therefore designed a quality-of-life instrument, the Cleveland Global Quality of Life (CGQL) instrument (Fazio Score), to fulfill these requirements, and we used this instrument to prospectively evaluate long-term quality of life in a large cohort of patients undergoing pelvic pouch construction with stapled IPAA. We also evaluated functional outcome and satisfaction with surgery in these patients. An important part of the study was the evaluation and validation of our novel quality-of-life instrument.
METHODS
Patients
All patients who underwent RPC with IPAA from December 1986 through June 1997 and who were followed for ≥12 months after surgery were included in the study. The first IPAA at the Cleveland Clinic was performed in December 1986. Ileal pouches were constructed using a J or an S configuration, but in all cases the pouch was stapled to the upper anal canal using a circular stapler inserted transanally. Patients were included in the study regardless of the indication for surgery.
Follow-up information was collected prospectively. Patients were required to complete a self-administered, structured questionnaire at each return visit to the office. If the patient did not attend the office for >1 year, the information was obtained by a mailed questionnaire.
Quality of Life Scale
Quality of life was assessed by the CGQL. Patients were asked to rate three items (current quality of life, current quality of health, and current energy level), each on a scale of 0 to 10 (0, worst; 10, best). The scores were added, and the final CGQL utility score was obtained by dividing this result by 30 (Fig. 1).
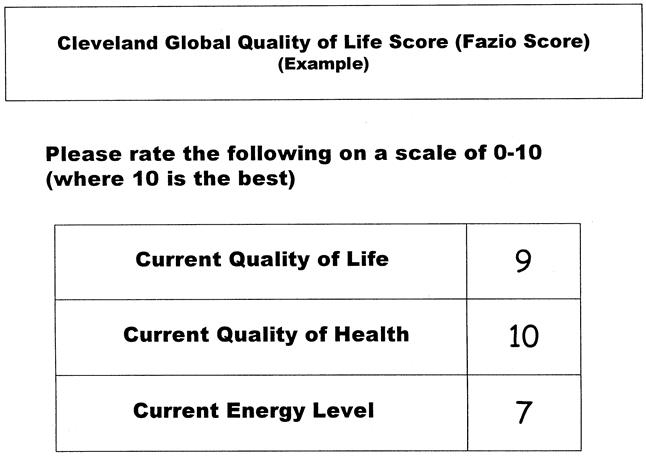
Figure 1. Sample Cleveland Global Quality of Life (CGQL) form. The patient is asked to score each of the three items in the right-hand column. In this case, the patient scored 9, 10, and 7 for the quality of life, quality of health, and energy level, respectively. These scores were added and the total divided by 30 to give a final score of 0.867 in this case.
Serial Quality of Life
Serial quality-of-life measurements were reported for the periods 0 to 2 years, 2 to 5 years, 5 to 8 years, and >8 years. If more than one questionnaire was completed by a patient during a particular period, the most recent information available was used to represent that interval.
Validation of Quality of Life Score
Reliability of the CGQL was determined by calculating the internal consistency between the three items of the scale using Cronbach’s alpha, 11,12 which, for acceptable internal reliability, should exceed 0.85. 7 Responsiveness was demonstrated by determining whether a significant deterioration or improvement in the number of bowel movements per 24 hours was associated with a change in the CGQL. The 50th and 90th percentiles for number of bowel movements per 24 hours were calculated using all follow-up data for all patients in the study. A significant deterioration in stool frequency was defined as an increase in frequency from below the 50th percentile at one follow-up visit to above the 90th percentile at the next consecutive visit. A significant improvement was defined as a reduction in stool frequency from above the 90th percentile to below the 50th percentile on consecutive visits. Construct validity was evaluated by determining whether fecal incontinence or restriction in work, social, or sexual activity was associated with a reduction in the CGQL. Validity was further demonstrated by correlating the CGQL with the Short Form 36 (SF-36) 7 quality-of-life scale in a subgroup of patients. The CGQL and the SF-36 were administered to a random subgroup of the overall study cohort. These tests were administered concurrently, and each patient selected was studied once only. Ideally, results from each quality-of-life scale were obtained at the same time, but in all cases they were obtained within a month of each other.
Satisfaction With Surgery
Patients were asked whether they would undergo the procedure again and whether they would recommend it to others. Satisfaction with surgery was also determined by asking the patient to score his or her “happiness with the surgery” on a scale of 0 to 10, with 10 representing complete satisfaction with the procedure.
Grading of Functional Outcome
Functional outcome was determined prospectively by questionnaire. Patients were asked whether they had incontinence for liquid or solid stool, and whether incontinence occurred never, sometimes, or always. Patients were also asked about pad usage, presence of fecal seepage, and the number of bowel movements during daytime or bedtime hours. The patients were also asked about the presence of urgency, restrictions of diet, social function, and sexual activity, as well as any work restrictions. As for quality of life, if patients completed the questionnaire more than once in any of the postoperative intervals, the most recent information was used to represent that interval.
Statistical Analysis
Data were analyzed using SPSS software (SPSS Inc., Chicago, IL). Analysis of variance was used to compare means, and Dunnett’s t test or Fisher’s least significant difference test was used for multiple comparisons. Linear regression and logistic regression were used using forward stepwise methods to determine factors associated with CGQL score or the occurrence of incontinence, respectively. In each of these models, time (postoperative interval) was included as a factor in the model in an attempt to address the assumption of independence in the regression analysis. Reliability analysis using the Cronbach’s alpha was used as described above. Correlation coefficients were calculated using Spearman’s test. Paired data for the responsiveness analysis were compared with the Wilcoxon signed rank test. Chi square test or Fisher’s exact test was used for categorical data. To reduce the possibility of type I statistical errors when these tests were used to test multiple planned comparisons, the experimentwise error rate (αEW) for each group of comparisons was maintained at 0.05 by setting the error rate per contrast (αPC) at 0.05/C where C was the number of contrasts tested. For all other statistics, the error rate (α) was set at 0.05, and no test was considered significant unless p < 0.05. Two-sided significance was obtained where appropriate.
RESULTS
A total of 1156 consecutive patients underwent RPC with IPAA from December 1986 through June 1997. Of these, 977 (85%) were followed for ≥12 months and were included in this study. Characteristics of patients at the time of surgery are given in Table 1. Patients were followed for a median of 5.0 years (range, 1.0 to 12.0). Patients were assessed on a median of four occasions (range, 1 to 13). This represented a total of 5118 patient-years of follow-up.
Table 1. PATIENT CHARACTERISTICS
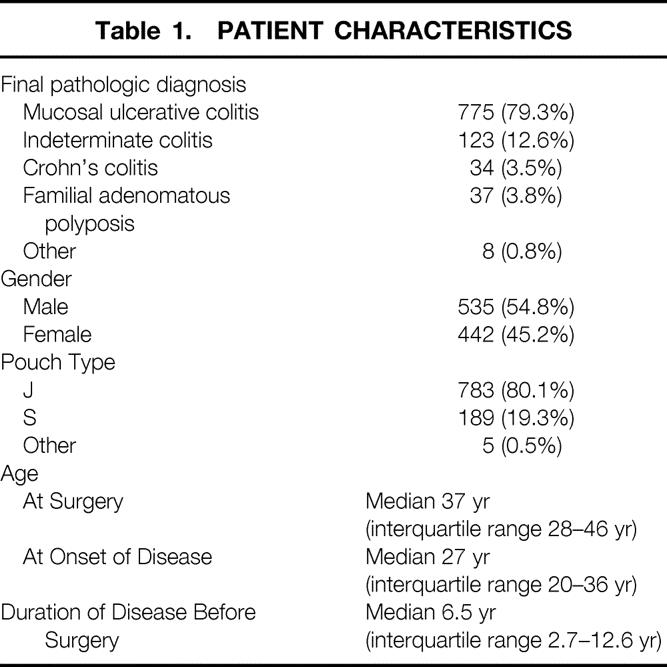
Nine pouches (0.9%) were excised a median of 4.9 years after surgery (range, 2.3 to 7.2). In addition, 11 patients (1.1%) had ileostomies at the most recent follow-up, a median of 5.1 years after surgery (range, 1.8 to 10.8). Thirteen patients (1.3%) underwent ileostomy some time after the pouch surgery but subsequently underwent reversal and were not diverted at the most recent follow-up.
Validation of CGQL Instrument
To determine reliability, Cronbach’s alpha was calculated using all 3366 questionnaires completed by the 977 patients in the study in which all three items were answered. Cronbach’s alpha for these data was 0.866, demonstrating acceptable internal reliability. For responsiveness analysis, the 50th and 90th percentiles for frequency of stool during a 24-hour period were calculated as 7 and 11, respectively.
Forty-one patients showed a deterioration in frequency of stool from ≤7 to ≥11 per 24 hours on consecutive visits. In one patient, this occurred on two occasions. The median CGQL was 0.83 (interquartile range [IQR] 0.77 to 1.0) on the first of these consecutive visits and 0.73 (IQR 0.63 to 0.88) on the subsequent visit where the deterioration was noted (p < 0.001, Wilcoxon signed rank test).
Sixty-seven patients showed a decrease in stool frequency from ≥11 to ≤7 stools per 24 hours. In one patient, this occurred on two occasions. In these patients, the median CGQL increased from 0.73 (IQR 0.67 to 0.83) to 0.83 (IQR 0.73 to 0.90; p < 0.001).
To evaluate validity of the CGQL further, CGQL and SF-36 scores were obtained on a random subgroup of 163 patients after pouch surgery. This sample was representative of the entire study cohort: the median age of the sample at the time of surgery was 37 years (IQR 29 to 45), the duration of disease before surgery was 7.6 years (IQR 2.6 to 13.6), and 55% of the sample were men. These forms were sent a median of 5.0 years (range, 1.0 to 10.0, IQR 3.0 to 7.0) after surgery.
In seven patients, one or two items of the SF-36 could not be calculated due to missing data. In two of these seven patients and in a further two patients in whom the SF-36 was complete, the CGQL could not be calculated for the same reason. The correlation coefficients between the items of the CGQL and the SF-36 are shown in Table 2. There is a significant correlation between each individual item as well as the total score of the CGQL and the individual item and total scores of the SF-36. The CGQL correlates very well with the vitality, general health, social functioning, and role physical items of the SF-36; the best correlation of all is between the CGQL and the overall SF-36 score.
Table 2. CORRELATION BETWEEN SF-36 AND CGQL
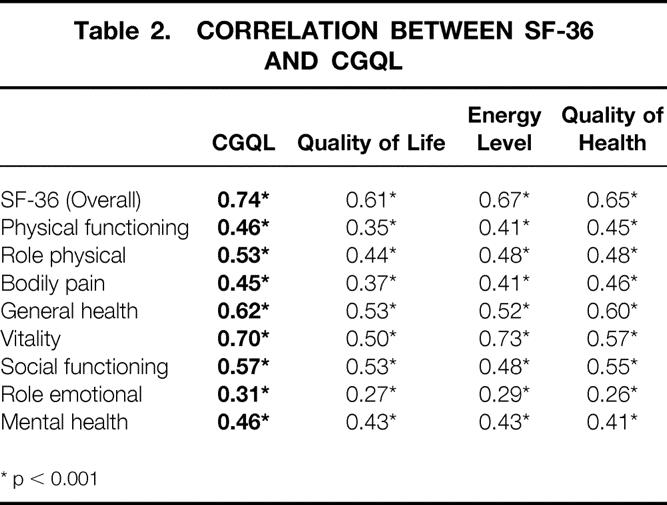
* p < 0.001
Quality of Life
Overall serial quality-of-life scores using the CGQL are shown in Figure 2. The overall CGQL score, as well as each of its three components, was significantly increased during the intervals 2 to 5 years, 5 to 8 years, and >8 years after surgery compared with the interval 0 to 2 years after surgery. There was no decrease in the quality of life with time after surgery.
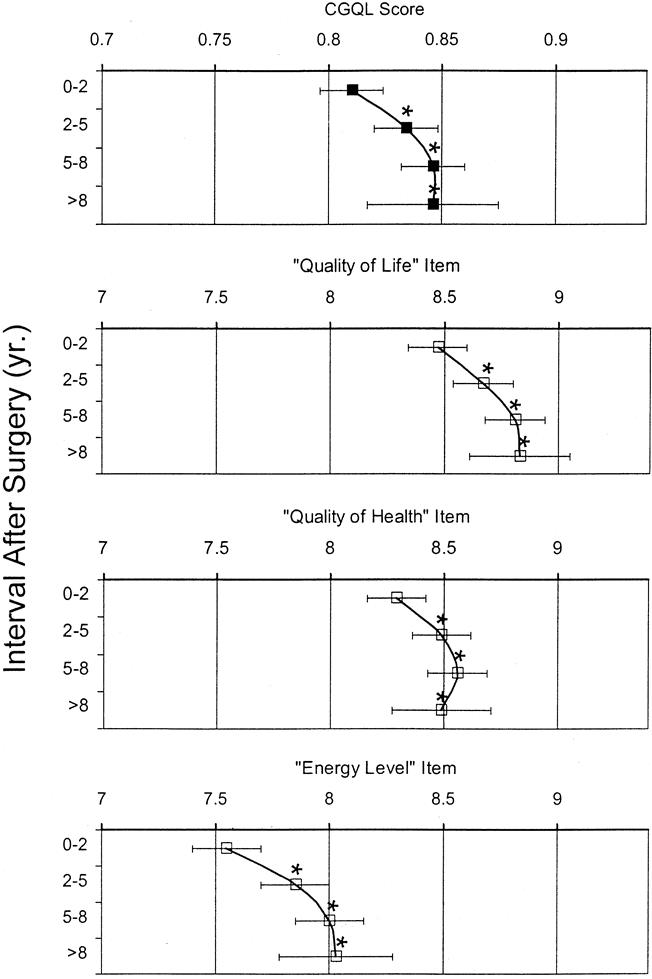
Figure 2. Overall Cleveland Global Quality of Life (CGQL) score and its three components (quality of life, energy level, and quality of health) for the periods 0 to 2 years, 2 to 5 years, 5 to 8 years, and >8 years after surgery. The overall CGQL score is scored on a scale of 0 to 1; each item is scored on a scale of 0 to 10. Each point represents a mean score; the black bars represent the 95% confidence intervals. For the overall CGQL and for each of its three component items, the scores significantly increased >2 years after surgery. *p < 0.05 vs. 0 to 2 years (analysis of variance with Dunnett’s test for multiple comparisons).
The SF-36 scores for the 163 patients in the random subgroup to whom this instrument was administered are shown in Figure 3. These scores compared well with the normalized mean values obtained for the general U.S. population. 7 The mean CGQL of this subgroup of patients in whom SF-36 scores were also obtained was 0.84 (95% confidence interval [CI] 0.81 to 0.86), compared with the mean CGQL calculated from all time points for all patients in the study of 0.83 (95% CI 0.82 to 0.84; p > 0.1).
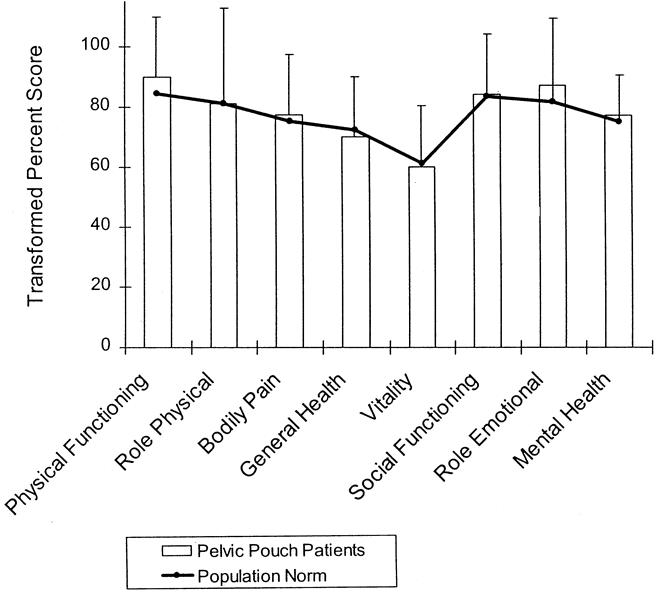
Figure 3. Scores for individual items of the SF-36 for 163 patients randomly selected from the study cohort. Scores are transformed and expressed on a scale of 0 to 100. Mean patient scores are shown by the shaded bars; standard deviations are shown by the error bars. Norms for the mean values for each item of the SF-36 for the general U.S. population are shown by the heavy black line. For both scales, higher scores represent a better quality of life. It is apparent that there is no difference between the scores of patients after pelvic pouch surgery and the general U.S. population for any of the eight items of the SF-36.
Functional Outcome
Table 3 shows the prevalence of incontinence as reported by the patients. When incontinence occurred, it was reported as occurring “sometimes” in most of the patients and was rarely reported as occurring always. There was a significant decrease in both the incidence of any reported incontinence (“always” or “sometimes”) and the incontinence reported “always” occurring immediately after surgery. After the early postoperative period (0 to 2 years), the incidence of incontinence reported “sometimes” showed some increase, but not to more than preoperative values.
Table 3. PREVALENCE OF INCONTINENCE
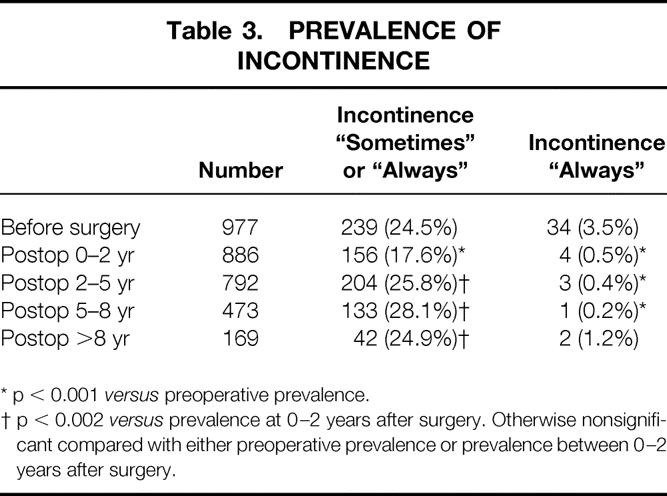
* p < 0.001 versus preoperative prevalence.
† p < 0.002 versus prevalence at 0–2 years after surgery. Otherwise nonsignificant compared with either preoperative prevalence or prevalence between 0–2 years after surgery.
The patterns of seepage and pad usage are shown in Figure 4. Use of pads either by day or by night was reported by 13% to 20% of patients; this was similar to the incidence of day seepage. Night seepage was more common, occurring in 28% to 31% of patients. There was no significant deterioration over time.
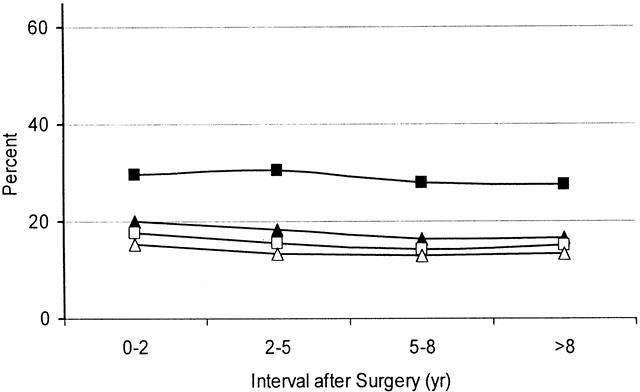
Figure 4. Percentage of patients with diurnal (□) or nocturnal (▪) seepage and/or diurnal (▵) or nocturnal (▴) pad usage during the four time periods studied after surgery. There were no significant changes in the incidence of seepage or pad usage with time after surgery.
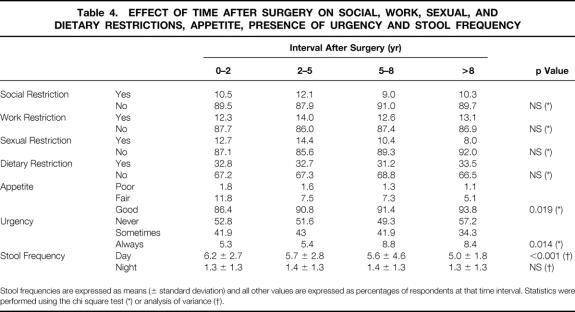
Stool frequency, urgency, appetite, and restriction of social activity, sexual activity, work, and diet are shown in Table 4. Of these factors, there was a relatively small but significant reduction in the number of daytime bowel movements, although nocturnal stool frequency remained stable during the duration of the follow-up. Appetite also improved significantly with time.
Table 4. EFFECT OF TIME AFTER SURGERY ON SOCIAL, WORK, SEXUAL, AND DIETARY RESTRICTIONS, APPETITE, PRESENCE OF URGENCY AND STOOL FREQUENCY
Stool frequencies are expressed as means (± standard deviation) and all other values are expressed as percentages of respondents at that time interval. Statistics were performed using the chi square test (*) or analysis of variance (†).
Factors Influencing Quality of Life
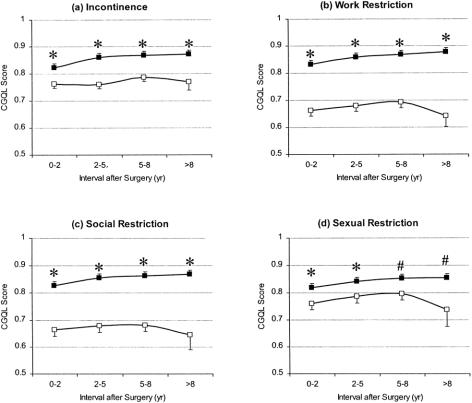
The effects of patient gender, age at surgery, duration of disease before surgery, and final pathologic diagnosis on quality of life are shown in Table 5. Of these, only age younger than 35 at the time of surgery predicted a good quality of life on univariate analysis. Figure 5 shows the relation between the presence of incontinence, work restriction, social restriction, and sexual restriction on CGQL at each of the four postoperative time intervals studied. On univariate analysis, the presence of any of these factors was associated with a relatively poor quality of life compared with patients without these complaints at each of the time intervals studied. There was a significant inverse correlation between the stool frequency and CGQL (r = −0.348, p < 0.001).
Table 5. EFFECT OF DIAGNOSIS AND DURATION OF DISEASE ON QUALITY OF LIFE
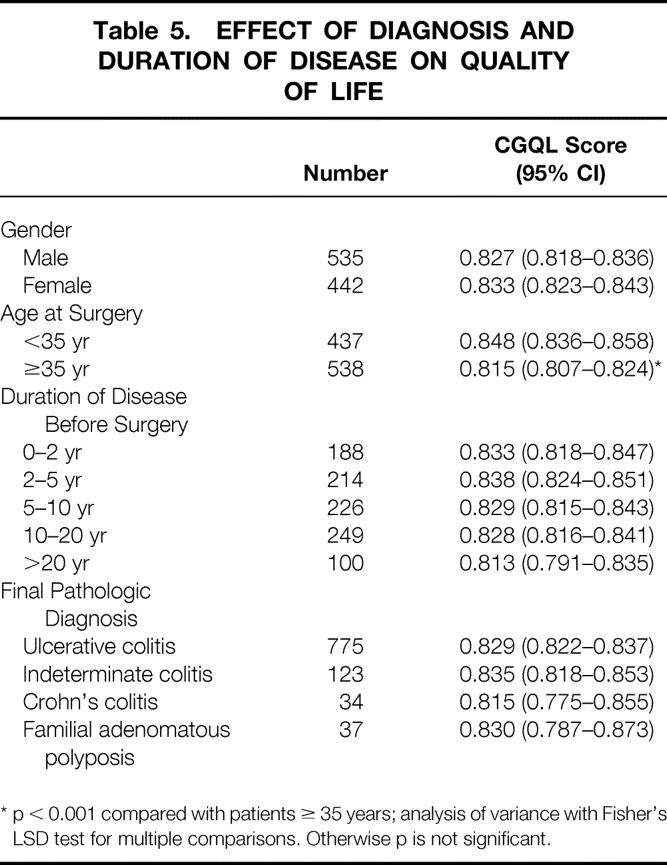
* p < 0.001 compared with patients ≥ 35 years; analysis of variance with Fisher’s LSD test for multiple comparisons. Otherwise p is not significant.
Figure 5. Quality of life as measured by the Cleveland Global Quality of Life (CGQL) in patients with (A) incontinence, (B) work restriction, (C) social restriction, and (D) sexual restriction. Open squares represent CGQL scores in patients with incontinence or work, social, or sexual restrictions; closed squares represent CGQL scores in patients without these complaints. Values are mean scores, and the error bars represent the standard errors of the means. Incontinence and work, social, or sexual restrictions all result in a significantly lower CGQL at each time point studied. *p < 0.001; #p < 0.05 (analysis of variance).
Using a multiple regression model, work restriction (β = −0.25, p < 0.0001), social restriction (β = −0.20, p < 0.0001), number of stools per 24-hour period (β = −0.18, p < 0.0001), urgency at any time (β = −0.09, p < 0.0001), incontinence at any time (β = −0.10, p < 0.001), and age (in years) at the time of surgery (β = −0.06, p = 0.015) were all independent factors associated with a low quality of life, as determined by the CGQL. Although restriction in sexual activity was associated with a reduced CGQL on univariate analysis, there was no association between sexual restriction and CGQL on multivariate analysis. Dietary restriction, patient gender, or the preoperative diagnosis of colitis versus familial adenomatous polyposis did not influence quality of life in this model.
Factors Associated With Incontinence
Using logistic regression analysis with a binary dependent variable (normal continence vs. incontinence “sometimes” or “always”), the following covariates were tested for their effect on continence after surgery: age at surgery, patient gender, original pathologic diagnosis (colitis vs. familial adenomatous polyposis), frequency of stool, and whether the patient ever complained of urgency. Of these factors, there was a significant association between incontinence and the presence of urgency (odds ratio 2.62, 95% CI 2.19 to 3.13), total number of bowel movements per 24 hours (odds ratio 1.1, 95% CI 1.07 to 1.14), and age at surgery (odds ratio 1.026, 95% CI 1.017 to 1.035; all p < 0.001). However, in this model, there was no significant effect of patient gender or pathologic diagnosis.
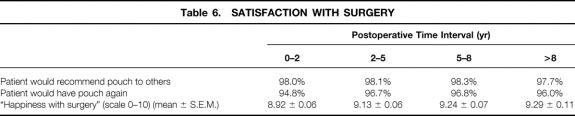
Satisfaction With Surgery
Measures of patient satisfaction with surgery are shown in Table 6. The great majority of patients would recommend their pouch to others and would themselves undergo the pouch surgery if the situation presented itself again. On a scale of 0 to 10, mean happiness-with-surgery scores for each of the four postoperative intervals studied ranged from 8.9 to 9.3. There was no reduction in patient satisfaction with time.
Table 6. SATISFACTION WITH SURGERY
DISCUSSION
With the technical advances in the procedure and the significant improvements in morbidity and mortality rates that have been achieved since the introduction of the ileal pouch surgery, quality of life and functional issues have become more important. In addition, because quality of life and functional status may change with time, it is important that these are studied at regular intervals after surgery. The present study shows that long-term quality of life is excellent and compares well with that of the general population in a very large group of patients studied prospectively after pelvic pouch construction.
Quality of life has been measured in many ways in patients after IPAA, but most studies have used either nonvalidated quality-of-life instruments or validated instruments in small numbers of patients at a single time point. 13–17 The present study is unique because, to our knowledge, it is the largest series in the literature to date that has a long duration of follow-up and that measures quality of life at multiple time points using a validated instrument. In addition, although previous large reports included a relatively high proportion of patients who underwent mucosectomy and hand-sewn anastomosis, the current study is confined to patients with a stapled IPAA, which in our opinion is now the procedure of choice unless specifically contraindicated. 18
A new outcome measure such as the CGQL must be shown to be valid, reliable, and responsive. A valid measure is one that measures what it is intended to measure. It has been shown previously that quality of life correlates with degree of continence 19; to demonstrate the construct validity of the CGQL, we hypothesized that patients with perfect continence would have a higher CGQL score than those with less-than-ideal continence. Further, we would expect patients with restrictions in work, social, or sexual function to have a reduced quality of life. All were associated with a reduced quality of life on univariate analysis, and all except sexual restriction were associated with a reduced CGQL on multivariate analysis. This confirms construct validity. Face validity, or the degree to which a test appears to measure what it is supposed to measure, is self-evident because of the simplicity and directness of the items of the CGQL. Validity was further demonstrated by showing that the overall CGQL score correlated well with the overall SF-36 score in a representative subgroup of patients. The SF-36 is a generic measure of quality of life derived from the Health Outcomes Study and measures quality of life under eight separate headings: physical functioning, social functioning, role limitation because of physical problems or because of emotional problems, mental health, vitality, physical pain, and general health. 7 It has previously been validated in patients after ileal pouch surgery. 17
The second prerequisite of a quality-of-life measure is responsiveness, or the sensitivity of the scale to measure change over time. We hypothesized that patients with a clinically significant increase in stool frequency from below the median of the group to above the 90th percentile would undergo a decrease in the quality of life, and vice versa. As expected, these changes in quality of life occurring with changes in stool frequency were identified using the CGQL, demonstrating the responsiveness of the instrument.
This study also showed the CGQL to be reliable. A reliable measure is one where similar measurements in a subject are similar in different settings. 11 One of the most widely used measures of reliability is the demonstration of internal reliability, which measures the extent to which different questions within a scale yield consistent responses. Using Cronbach’s alpha, the CGQL demonstrates a high level of internal consistency, with an α value of 0.87; this exceeds the accepted threshold for reliability, which ranges from 0.7 to 0.85. 7,20
Unlike some of the more complex generic health-related quality-of-life indicators such as the SF-36, the Sickness Impact Profile, or the inflammatory bowel disease-specific indicators such as the IBDQ or the RFIPC, 6,7,9,10 the CGQL is a global measure of quality of life and does not attempt to differentiate psychosocial from disease-related factors. In general, utility-based measures such as the CGQL are less sensitive than psychometrically based methods 21 and may lack the sensitivity to demonstrate the small differences in quality of life in an individual patient that more complex, disease-specific measures may show. Although the CGQL may discriminate between patients with good or bad quality of life and may also demonstrate the changes in quality of life related to specific modifying factors or over time, the clinical significance of an absolute CGQL score value requires further study. In the present study, the score has been shown to reflect physical and social outcome best, and emotional outcome to a lesser extent.
Nevertheless, there is no gold standard to measure quality of life in patients after pelvic pouch surgery, and all validated methods have advantages and disadvantages. The CGQL has the great advantage of its simplicity and ease of self-administration, particularly for repeated measures in large groups of patients. Like the time trade-off technique and the direct questioning of objectives technique, which are the other utility measures commonly applied in patients with inflammatory bowel disease and those who undergo pouch surgery, 16,17 the CGQL also has an advantage that the absence of very specific questions may avoid introducing a certain source of bias into the quality-of-life score. For example, use of increased stool frequency as an indicator of poor quality of life in patients with inflammatory bowel disease may bias the scale in favor of medical therapy; five or six bowel movements per day may be associated with disease activity and poor quality of life in medically treated patients with ulcerative colitis but may be irrelevant in patients with ileostomy and may be very well tolerated and associated with an excellent quality of life in patients with a pelvic pouch, as shown in the current study.
The CGQL instrument may be criticized for the ambiguity of the questions, but it was precisely with this vagueness that we wanted to design the items. Just as in the direct questioning of objectives method, where the patient chooses and assigns weights to the three questions based on his or her quality of life objectives, 22 the nonspecific and nondirective nature of the CGQL questions allow the patient to decide what is relevant and important within each component. Thus, by allowing wide latitude to the patient in defining the important quality-of-life considerations, the CGQL instrument may achieve similar objectives to the direct questioning of objectives method (i.e., relevance to the individual patient), but with a simpler and more easily administered scoring system.
The calibration of the CGQL with the SF-36 in the present study showed that overall postoperative quality of life is comparable with that of the general population. A small study from Duke University Medical Center using the SF-36 also found no differences between patients after pelvic pouch surgery and a normal population. 17 Using their own quality-of-life indicators, Kohler et al also showed no difference between postoperative quality of life after pelvic pouch surgery and after cholecystectomy. 23 Using a quality-of-life instrument that they formulated and validated in patients with inflammatory bowel disorder, Martin et al demonstrated that quality of life in 29 patients after IPAA was similar to that of unoperated patients with ulcerative colitis in remission or with mild disease activity. 15 Their finding that patients with IPAA had a lower quality of life than healthy controls differed from our study. One reason may be that the single highest score in the quality-of-life measurement that they used was for emotional function, whereas emotional function may be among the least important components of the CGQL. Nevertheless, in the subgroup of our patients tested with the SF-36, the mean emotional functioning score was just above that for the mean of the general population. Other authors have confirmed the high quality of life after IPAA using nonvalidated questionnaires. 14,24
Much of the improvement in quality of life after surgery may result from the removal of the diseased colon as well as the reduction in cancer risk perceived by the patient rather than the actual reconstruction, 16 although Kohler et al reported that quality of life was best after pelvic pouch compared with Brooke ileostomy or Koch continent ileostomy. 25 However, the objective of the current study was not to determine the change in quality of life with surgery, but rather to determine what factors influence quality of life after the procedure. For the first time, we have shown in this study that quality of life is maintained over a long period of time after surgery. Using the Psychosocial Adjustment to Illness Scale and other measures, Weinryb et al showed that there was no further improvement in quality of life after closure of the ileostomy, but the postileostomy measurement was performed a median of 17 months after closure. 26 In the present study, the first time interval studied was 0 to 2 years, and the median interval between the pouch construction and the follow-up episode corresponding to this interval was 16 months (IQR 12 to 20). Our data show a significant improvement in quality of life after that time. We specifically did not study an earlier time interval because we wanted to exclude the acute effects of surgery in the analysis of long-term follow-up. Nevertheless, it would appear that there still may be a delayed effect of the procedure and adjustment to the pouch on the quality of life in the first 2 years. In fact, if we had studied the interval 0 to 1 years using the criteria outlined in the methods, the mean CGQL score during that time would have been 0.80 (95% CI 0.78 to 0.81) compared with 0.82 (95% CI 0.80 to 0.83) for the interval 1 to 2 years (p = 0.07). Although incontinence, urgency, work restriction, and social restriction all correlated negatively with quality of life, these did not decrease with time, even though quality of life improved. Some of this improvement in quality of life in the long term may be the result of increased coping mechanisms. 27
This series confirmed the findings of other large series showing a high level of patient satisfaction with pelvic pouches. 14,25 As it was for quality of life, this satisfaction was maintained over time.
Between 18% and 28% of patients had some degree of fecal incontinence during the different postoperative intervals studied. However, 24.5% had incontinence before surgery, with a 3.5% rate of major incontinence. This is similar to the Mayo Clinic experience. 13 As with other studies that report a similar incidence of incontinence, 28,29 incontinence in most of our patients was intermittent, and most of the patients did not wear pads. The incidence of incontinence “sometimes” or “always” appears to increase after the first few years after surgery. The reason for this is unclear. One possible factor is a decrease in anal function with advancing age, 30,31 although Takao et al have shown that age at the time of surgery does not influence functional outcome after pouch surgery. 32 We have previously reported on the effect of factors such as pouch size and compliance, internal and external sphincter tone, stool consistency, and the completeness of evacuation on fecal continence, 33 but these factors were not addressed in the current study. Nevertheless, even at >8 years after surgery, the prevalence of incontinence in the current study was no higher than that reported before surgery.
More than 40% of patients reported urgency during each of the postoperative time intervals. We have reported previously an incidence of this condition of 23.5%, 3 and an incidence of 31% was reported by the Mayo Clinic. 34 Although not all cases of urgency in the present series are the result of pouchitis, occasional pouchitis may represent a significant proportion. Some of the cases may also be the result of inflammation of the retained anal transitional zone. 35 However, in most of our cases the attacks of urgency were short-lived, with constant urgency reported in a small percentage.
In summary, this series represents a very large number of patients followed for a long period of time. We have shown that in this tertiary referral center, long-term functional results are satisfactory and quality of life is excellent after RPC for colitis or familial adenomatous polyposis. Further, we have shown that the CGQL is a valid and reliable way of measuring quality of life after pelvic pouch surgery.
Acknowledgment
The authors thank Ms. J. Bast, Mr. Dino Paulesc, and Ms. Dianne Rudzitis, Department of Colorectal Surgery, for help with data collection and Dr. Michelle Secic, Department of Biostatistics, for reviewing the manuscript and for statistical advice.
Discussion
Dr. David A. Rothenberger (St. Paul, Minnesota): I enjoyed your paper as it really reflects the maturation of ileal pouch anal anastomosis surgery. We have learned how to do this operation with reasonable safety, and the focus is now turning appropriately to optimizing and assessing function. The authors are to be congratulated for this effort, which goes back many years and looks at function in a prospective manner in a large, consecutive series of patients who have undergone a double-stapled nonmucosectomy ileal pouch. My reading of the manuscript prompts several questions.
First, did you review a subgroup of patients who had pelvic septic complications? What was their ultimate quality of life? Did your instruments detect any kind of problems in that subgroup?
Secondly, could you comment on the somewhat complex relationship between stool frequency and use of antidiarrheal medications and quality of life? We have struggled with trying to understand this complex relationship.
Thirdly, what percentage of your patients, who are generally young, reported sexual dysfunction? Apparently, sexual dysfunction did not seem to be an important variable in this quality-of-life analysis.
Fourth, have you used the same sort of analysis to determine whether functional results would vary with the operative procedure performed? Specifically, have you assessed patients who have had a mucosectomy and a handsewn ileal pouch anal anastomosis, as opposed to a double-stapled nonmucosectomy pouch?
Fifth, have you assessed patients who have elected to undergo a total proctocolectomy and a Brooke ileostomy rather than an ileal pouch procedure? Certainly in our practice after full informed consent, a significant number of our patients still opt to have a permanent ileostomy rather than an ileal pouch, even though we have offered that option to them. Our impression, though not validated in the way you have, is that these patients remain extremely pleased with their quality of life. They are free of their disease. Their life gets back to normal function. Have you looked at that subgroup of patients?
Presenter Dr. Michael G. O’Riordain (Cleveland, Ohio): With regard to sepsis, about 8% of the patients in this study did have sepsis postoperatively. But there was actually no difference in quality of life between these patients and the patients who didn’t have sepsis postoperatively as determined by the CGQL score. I must point out that at the 0- to 2-year time interval, the data was collected at a median of 16 months after surgery. So that was probably well after the time of the sepsis.
With regards to antidiarrheal medication and stool frequency, this is very interesting because in our study we have shown that although there was a slight but significant drop in the stool frequency from seven stools per day down to about six stools per day during the duration of the study, this is paralleled by a huge decrease in the usage of antidiarrheal medications from about 80% down to about 20% during the study. Therefore, pouch adaptation is probably more reflected with the reduction in antidiarrheal medication rather than the reduction of stool frequency. We didn’t include antidiarrheals in our multivariate analysis, but on univariate analysis, antidiarrheals are actually associated with a poorer quality of life compared with patients who weren’t on antidiarrheals for each of the time intervals studied. Presumably, this is unlikely to be due to the antidiarrheals themselves and is multifactorial and probably due to things like stool frequency, which are increased in that subgroup of patients.
The next question as to sexual dysfunction: About 12% of our patients described sexual dysfunction during the period of the study. However, we didn’t specify what sexual dysfunction means, and this may vary from severe impotence to maybe concerns about whether there might be some leakage during the sexual act or whatever. Our results are actually similar to Mayo’s results which have been reported by Kohler et al. Using very similar questions, they got about a 14% incidence of sexual dysfunction. We have previously reported that the incidence of serious sexual dysfunction is very low in these patients. The incidence of impotence is actually well under 1% and the incidence of retrograde ejaculations under 5%. This may partly explain why on multivariate analysis, there is no correlation between the broader definition of sexual dysfunction in this study and poor quality of life.
The fourth question you asked was regarding mucosectomy. This is very interesting. First of all, I should explain why we excluded mucosectomy from this series of patients. As you know, mucosectomy was initially reported with the first description of the pouch in 1978, and it was only in the mid-1980s that the stapled anastomosis was described. Most series actually have a large percentage of mucosectomy patients in their reports. However, we now believe that the stapled anastomosis is the procedure of choice for most patients unless there is a specific contraindication, and in fact in the last year of this study, 1996, of the 158 pouches which we performed, only 17 had a mucosectomy and handsewn anastomosis. So we wanted to restrict the study to what we now consider the procedure of choice in most patients.
Overall, the quality of life is no different between mucosectomy and stapled anastomosis. But interestingly, in the 0 to 2 years interval, the patients with mucosectomy have a slightly increased quality of life compared with the patients who had double-stapled anastomosis, but over the period of follow-up, the quality of life in the mucosectomy patients dropped slightly, so that between 5 and 8 years-plus, the quality of life in mucosectomy patients is actually less than the quality of life in the double-stapled patients. There are also differences in continence between mucosectomy and stapled anastomosis. In fact, patients with mucosectomies have increased reported incontinence, increased seepage day and night, increased pad usage day and night, compared with patients with double-stapled anastomosis.
The final question you asked was about whether we had applied this method to Brooke ileostomy patients. Of course this would be a very nice thing to do, but we actually didn’t do it. There are other studies, particularly from Mayo and Toronto, which have tested this and, just as with your data, have shown that there are no real differences between quality of life in patients who had a Brooke ileostomy, pelvic pouch, or indeed continent ileostomy. In fact it is likely that the main improvements in quality of life after pouch surgery are actually due to the colectomy rather than the actual pouches themselves. But that wasn’t the purpose. Our purpose wasn’t to look at perioperative changes and quality of life. The purpose of our study was to determine what the long-term quality of life is over time and what factors influence that.
Dr. Robin S. McLeod (Toronto, Canada): I too would like to congratulate the authors on a very good paper. I think the results are impressive simply as a clinical series with a large number of patients and excellent surgical results. In addition to this, the authors should be congratulated for actually looking at quality of life because obviously this is extremely important in this cohort of patients. So I congratulate you for that and for your attempt to develop a quality-of-life index.
This is important because, to date, most authors have evaluated outcome based on functional results, that is, the number of bowel movements per 24 hours or incontinence. And as we know, function does not necessarily correlate with quality of life. I do have a few questions, however, and most of those are related to the development of your index.
The first question is that I think the greatest value of this index would be as a discriminative tool, that is, to differentiate outcome among people who had this operation. Similar to Dr. Rothenberger’s question, we know that there are certain people who don’t do as well, whether it is from pelvic sepsis or whatever, and I didn’t really see that you have looked at discriminative validity to see whether the tool was valid in this way.
You did look at responsiveness, but only in the subgroup of 60 patients, and I wondered if you looked at it in the total cohort of patients. And with regards to that, I wondered why you looked at it by dichotomizing the data into the two centiles rather than looking at it as continuous data.
You should be congratulated for making a rather easy-to-use instrument that can be applied. Often, these quality-of-life indexes can be quite cumbersome, so people don’t use them. But I wondered how you chose the three questions that you did. Secondly, I have a bit of a concern that at least two out of the three sample the physical domain and I wonder whether or not you really are measuring quality of life or whether this index is really just a functional score rather than a quality-of-life measure? Did you consider validating your index against some other validated psychosocial instruments to see whether there was validity in that regard?
Finally, I have questions regarding the use of the SF-36. In the slide, I saw that there was quite a high correlation coefficient of .74. But in the manuscript, the correlation coefficients didn’t seem to be that high, most of them were in the order of .4 or .5 for the subscales. Although you say that is a good correlation, that is really fair to maybe moderate correlation. I think those are fairly low, and I would have expected them to be a little bit higher. I wondered if you have concerns about that with regards to your index.
But again having said all of those comments, I think that it is a very good effort and a worthwhile contribution, and I appreciate having had the opportunity of reading it.
Dr. O’Riordain: We have shown that the CGQL can discriminate between study subgroups, showing differences in quality of life between patients with or without incontinence, work restriction, or social restriction.
With regard to responsiveness, the reason we dichotomized the data was because significant changes in stool frequency have been associated with altered quality of life in the past, and I think that this is a reasonable thing to do. We expected changes to be seen in the quality of life with these changes in stool frequency, and these were actually shown in both directions. Forty-one patients had an increase in stool frequency, and 67 patients, I think, had a reduction in stool frequency, and in each case the expected quality-of-life changes occurred. When you look at the whole sample, you see there is actually no significant change in the quality of life in patients who didn’t show a significant increase or decrease in stool frequency. If you look at responsiveness using continuous data, the results are the same.
The third question you asked was how we chose these questions. What we wanted was a simple, easy-to-administer, valid, and reliable tool. These have face validity. It is quite clear to people that the three items of the scale asking about quality of life and quality of health and energy level are determining what they are actually asking for. However, the questions must be loosely defined so that it is the patient who decides what is important in quality of life or health, etcetera. There is a high correlation between the CGQL and the overall SF-36 score. CGQL is not simply a functional scale and actually correlates better with vitality and indeed social functioning than physical function. When you look at the correlation coefficients between the three items of the CGQL scale and the eight items of the SF-36 scale, there is relatively poor correlation with emotional outcome. It may be something that we will look at in future modifications of the CGQL score.
There were two reasons that we selected the generic psychometric test rather than a disease-specific psychometric test. One, we think that the CGQL is applicable to many situations other than pouch surgery and indeed other than inflammatory bowel disease altogether. Two, we are slightly concerned about applying scores such as the IBDQ in a condition which might be related to the situation where it was originally described where it isn’t actually the same. For example, using the IBDQ, a stool frequency of six would be considered in a medically treated patient to be associated with a poor quality of life because it would be associated with high disease activity; in a patient with an ileostomy, it might not be relevant at all, and in a patient with pelvic pouch, a stool frequency of six per day might be associated with very good quality of life, as we have shown in our study.
With regard to correlation coefficient, I think I answered that earlier. Of course, high correlation with other psychometric scores is not absolutely necessary for validity, because the scores may be measuring slightly different aspects of quality of life.
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): It seems a little paradoxical that your nocturnal seepage increased over 8 years and your satisfaction score paralleled that. Does that suggest a certain insensitivity of the Cleveland satisfaction score?
Dr. O’Riordain: I think a large number of items influenced the quality of life, and not least, patient expectations. I think that patient expectations probably do change with time. And this may change quality of life. I think our definition of quality of life is a function of the patient in relation to patient expectations.
Dr. Keith A. Kelly (Scottsdale, Arizona): I have one comment and a question. The comment is that your data on quality of life and ours at Mayo are very similar. But we did find that these pelvic pouch operations do improve quality of life over Brooke ileostomy and Koch pouch, especially for close physical encounters, like contact sports and sexual activity. So, I believe the quality of life with the pouch operation is the best of the three procedures.
My question relates to the functional outcome you mentioned. I notice that an increase in the amount of incontinence occurred as time passed after the operation. What are your thoughts as to the reason for this?
Two explanations occur to me. One is that, as patients age, the strength of their anal sphincter begins to decline. Is the increasing incontinence related to the strength of the sphincter, and have you done sphincter manometric measurements to test that? Another possibility is that the small amount of diseased mucosa in the proximal anal canal that you leave in place may lead to anal strictures or other anorectal problems that can lead to incontinence. Do you “scope” your patients to monitor this? Do you recommend that monitoring be done to look for, not only inflammatory changes, but also premalignant changes?
Dr. O’Riordain: With regard to the slight increases in incontinence with time, first of all, the reported incontinence with time is no different at any time period postoperatively compared with preoperative incontinence, apart from the initial postoperative period, when in fact the incidence of incontinence is actually less 2 years after surgery compared with preoperatively.
However, there does seem to be a trend upwards, as you point out. We agree that this is probably due to an aging process. As you know, Dr. Wexner’s group has shown recently that patients who have pouches at an older age do not have reduced functional outcome compared with patients who have pouches at a younger age. But we still believe that there is an aging process in this, and I think this is why some of the patients may develop slightly reduced continence in the longer term.
I must point out what we mean by reported incontinence. All we are saying is that these patients report incontinence sometimes. This may be once a month or may be very rarely. This is important. It is not that these patients are all wearing diapers and that they are severely incontinent. I don’t think they are. There is a very good quality of life even in many patients with some incontinence.
With regard to mucosectomy, we obviously leave the mucosa by definition in this operation. I think this is associated with some degree of strip colitis, and I think our group has shown that the strip colitis rate is around 20%. This may explain some of the urgency in our patients. In fact, we haven’t shown the data here, but urgency is reported sometimes in these patients up to about 40% of cases at all time periods postoperatively. And some of this may actually be due to strip colitis. However, interestingly, if you look at the mucosectomy patients, they have reduced urgency compared to stapled anastomosis in the early time postoperatively, but later there is no difference in urgency between mucosectomy patients versus stapled patients. In fact, the urgency rate may even be slightly higher in the mucosectomy patients later postoperatively.
With regard to your last question, clearly we do recommend monitoring of the anal transitional zone, particularly for dysplastic changes. We presented data on that last year. And we certainly recommend long-term follow-up for the development of dysplasia in these patients.
Dr. Christian Herfarth (Heidelberg, Germany): You mix the patients with ulcerative colitis and polyposis, but the follow-up of those patients is quite different concerning the criteria. Did you stratify the patients with polyposis and ulcerative colitis, and what was the difference between those patient groups?
Dr. O’Riordain: That is a very good question. In fact, the percentage of FAP in this paper is slightly less than the percentage of FAP in our previous papers. That is because FAP patients tend to be more likely to have a mucosectomy than ulcerative colitis patients. However, we have over 30 patients in this study with polyposis. When you use ulcerative colitis versus FAP as one of the factors in your multivariate analysis, there is no difference in quality of life between patients with FAP or patients with ulcerative colitis.
Footnotes
Correspondence: Victor W. Fazio, MD, Dept. of Colorectal Surgery, Cleveland Clinic Foundation, 9500 Euclid Ave., Desk A-111, Cleveland, OH 44195.
Presented at the 119th Annual Meeting of the American Surgical Association, April 15–17, 1999, Hyatt Regency Hotel, San Diego, California.
Accepted for publication April 1999.
References
- 1.Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J 1978; ii:8:5–88. [DOI] [PMC free article] [PubMed]
- 2.Fazio VW. Inflammatory bowel disease: surgical aspects. In Farmer RG, Achtar E, Fleshler B., eds. Clinical gastroenterology. New York: Raven Press; 1983: 361–373.
- 3.Fazio VW, Ziv Y, Church JM, et al. Ileal pouch-anal anastomoses:: complications and function in 1005 patients. Ann Surg 1995; 222: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly KA. Anal sphincter-saving operations for chronic ulcerative colitis. Am J Surg 1992; 163: 5–11. [DOI] [PubMed] [Google Scholar]
- 5.Wexner SD, Jensen L, Rothenberger DA, Wong WD, Goldberg SM. Long-term functional analysis of the ileoanal reservoir. Dis Colon Rectum 1989; 32: 275–281. [DOI] [PubMed] [Google Scholar]
- 6.Bergner M, Bobbitt RA, Carter WB, Gilson BS. The sickness impact profile: : development and final revision of a health status measure. Med Care 1981; 19: 787–805. [DOI] [PubMed] [Google Scholar]
- 7.Ware JE. SF-36 Health Survey Manual and interpretation guide. Boston: The Medical Outcomes Trust; 1993.
- 8.Farmer RG, Wesley KA, Farmer JM. Quality of life assessment by patients with IBD. Clev Clin J Med 1992; 59: 35. [DOI] [PubMed] [Google Scholar]
- 9.Irvine EJ, Feagan B, Rochon J, et al. Quality of life:: a valid and reliable measure of of therapeutic efficacy in the treatment of IBD. Gastroenterology 1994; 106: 287–296. [DOI] [PubMed] [Google Scholar]
- 10.Drossman DA, Leserman J, Li ZM, Mitchell CM, Zagami EA. The rating form of IBD patient concerns: : a new measure of health status. Psychosom Med 1991; 53: 701–712. [DOI] [PubMed] [Google Scholar]
- 11.Streiner GL, Norman DR. Health measurement scales: a practical guide to their development and use. Oxford: Oxford University Press; 1990.
- 12.Cronbach LJ. Co-efficient alpha and the internal structure of tests. Psychometrica 1951; 16: 297–334. [Google Scholar]
- 13.Pemberton JH, Phillips SF, Ready RR, Zinmeister AR, Beahrs OH. Quality of life after Brooke ileostomy and ileal pouch anal anastomosis. Ann Surg 1989; 209: 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcello PW, Roberts PL, Schoetz DJ Jr, Coller JA, Murray JJ, Veidenheimer MC. Long-term results of the ileo-anal pouch procedure. Arch Surg 1993; 128: 500–504. [DOI] [PubMed] [Google Scholar]
- 15.Martin A, Dinca M, Leone L, et al. Quality of life after proctocolectomy and ileo-anal anastomosis for severe ulcerative colitis. Am J Gastroenterol 1998; 93: 166–169. [DOI] [PubMed] [Google Scholar]
- 16.McLeod RS, Churchill DN, Lock AM, Vanderburgh S, Cohen Z. Quality of life of patients with ulcerative colitis pre-operatively and post-operatively. Gastroenterology 1991; 101: 1307–1313. [DOI] [PubMed] [Google Scholar]
- 17.Provenzale D, Shearin M, Phillips-Bute BG, et al. Health-related quality of life after ileoanal pull-through evaluation and assessment of new health status measures. Gastroenterology 1997; 113: 7–14. [DOI] [PubMed] [Google Scholar]
- 18.Fazio VW. What is the better surgical technique in ileo-pouch anal anastomosis? Stapled anastomosis. Inflam Bowel Dis 1996; 2: 148–150. [PubMed] [Google Scholar]
- 19.DeSouza CM, McLeod RS, Baxter N, O’Connor B, Ng E, Cohen Z. Quality of life after pelvic pouch procedure [abstract]. Can J Surg 1995; 38: A24. [Google Scholar]
- 20.Nunnally JC. Psychometric theory. New York: McGraw-Hill; 1978.
- 21.McLeod RS, Baxter NN. Quality of life of patients with inflammatory bowel disease after surgery. World J Surg 1998; 22: 375–381. [DOI] [PubMed] [Google Scholar]
- 22.Detsky AS, McLaughlin JR, Abrams HB. Quality of life in patients on long-term total parenteral nutrition at home. J Gen Intern Med 1986; 1: 26–33. [DOI] [PubMed] [Google Scholar]
- 23.Kohler LW, Pemberton JH, Hodge DO, Zinmeister AR, Kelly KA. Long-term functional results and quality of life after ileal pouch anal anastomosis and cholecystectomy. World J Surg 1992; 16: 1126. [DOI] [PubMed] [Google Scholar]
- 24.Pezim ME, Nicholls RJ. Quality of life after restorative proctocolectomy with pelvic ileal reservoir. Br J Surg 1985; 72: 31. [DOI] [PubMed] [Google Scholar]
- 25.Kohler LW, Pemberton JH, Zinmeister AR, Kelly KA. Quality of life after proctocolectomy: : a comparison of Brooke ileostomy, Koch pouch and ileal-pouch anal anastomosis. Gastroenterology 1991; 101: 679. [PubMed] [Google Scholar]
- 26.Weinryb RM, Gustavsson JP, Liljeqvist L, Poppen B, Rossel RJ. A prospective study of the quality of life after pelvic pouch operation. J Amer Coll Surg 1995; 180: 589–595. [PubMed] [Google Scholar]
- 27.Oresland T, Fasth S, Nordgren S, Akervall S, Hulten L. Pouch size:: the important functional determinant after restorative proctocolectomy. Br J Surg 1990; 77: 265–269. [DOI] [PubMed] [Google Scholar]
- 28.Romanos J, Samarasekera DN, Stebbing JF, Jewell DP, Kettlewell MG, Mortensen NJ. Outcome of 200 restorative proctocolectomy operations: : the John Radcliffe Hospital experience. Br J Surg 1997; 84: 814–818. [PubMed] [Google Scholar]
- 29.Pemberton JH, Kelly KA, Beart RW Jr, Dozois RR, Wolff BG, Ilstrup DM. Ileal pouch-anal anastomosis for chronic ulcerative colitis. Ann Surg 1987; 206: 504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jameson JS, Chia YW, Kamm MA, et al. Effect of age, sex and parity on anorectal function. Br J Surg 1994; 81: 1689–1692. [DOI] [PubMed] [Google Scholar]
- 31.Laurberg S, Swash M. Effects of aging on the anorectal sphincters and their innervation. Dis Colon Rectum 1989; 32: 737–742. [DOI] [PubMed] [Google Scholar]
- 32.Takao Y, Gilliland R, Nogueras JJ, Weiss EG, Wexner SD. Is age relevant to functional outcome after restorative proctocolectomy for ulcerative colitis? Prospective assessment of 122 cases. Ann Surg 1998; 227: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuckson WB, Fazio VW. Functional comparison between double and triple ileal loop pouches. Dis Colon Rectum 1991; 34: 17–21. [DOI] [PubMed] [Google Scholar]
- 34.Lohmuller JL, Pemberton JH, Dozois RR, et al. Pouchitis and extraintestinal manifestations of inflammatory bowel disease after ileal pouch-anal anastomosis. Ann Surg 1990; 211: 622–627. [PMC free article] [PubMed] [Google Scholar]
- 35.Lavery IC, Sirimarco MT, Ziv Y, Fazio VW. Anal canal inflammation after ileal pouch-anal anastomosis. The need for treatment. Dis Colon Rectum 1995; 38: 803–806. [DOI] [PubMed] [Google Scholar]