Abstract
Objective
To describe the technique and results of an alternative colon interposition procedure in which the ascending and transverse colon is used as graft, but that still relies on the left colonic artery for blood supply.
Summary Background Data
The standard procedure to obtain a left colon interposition graft requires ligation of the middle colic artery and mobilization of the left and right flexure. This approach carries a risk because preparation of the left flexure may damage arterial or venous collaterals located at this site that are crucial for graft perfusion.
Methods
The authors modified the standard technique so that mobilization of the left flexure is no longer necessary. To obtain a colon interposition graft that is long enough, the ascending colon was included into the graft by ligating the middle and the right colic artery. The left colic artery remained the blood-supplying vessel. From January 1997 to June 1998, 15 patients underwent modified colon interposition with a cervical anastomosis (12 esophagectomies, 3 esophagogastrectomies).
Results
In all cases, intraoperative blood supply from the left colic artery to the proximal ascending colon was sufficient. After surgery, four major complications occurred (27%). Endoscopy demonstrated a vital graft in all patients. In one patient a leakage of the cervical anastomosis was observed. One patient died of herpes pneumonia. Postoperative artificial ventilation was required for an average of 2.8 ± 4.6 days, the average intensive care unit stay was 6.9±4.5 days, and the average total hospital stay was 24.1 ± 15.1 days.
Conclusion
An intact left colic artery, including its collaterals at the splenic flexure, supplies sufficient blood to the proximal ascending colon after central ligation of the middle and right colic artery. Even without mobilization of the left flexure, a sufficient graft length can be obtained. Preliminary complication rates with the use of this technique for colon interposition are in the range of those found for the standard colon interposition technique. These modifications may represent an alternative to established procedures for creating a colon interposition graft.
Besides the stomach, the colon is considered a well-functioning and durable esophageal substitute. For esophageal reconstruction, an isoperistaltic colon graft should be used because an antiperistaltic reconstruction may be associated with significant spasms. 1 With the left colon, it is possible to obtain, in addition to an isoperistaltic reconstruction, the most extensive mobility of the graft. The standard procedure to obtain a left colon interposition graft includes ligation of the middle colic artery and mobilization of the left and the right flexure. The ascending branch of the left colic artery is also prepared and isolated. Then, the mesentery close to the colon and the terminal arteries at the site of the left flexure are transected to achieve the required mobility of the graft. 1 For esophageal reconstruction, the large bowel is transected at the right flexure, which is passed behind the stomach and through the hiatus to create a mediastinal interposition with an esophagocolonic anastomosis. 2 The reconstruction is completed after transection of the proximal portion of the descending colon and after anastomosing the graft to the stomach.
This above procedure carries a risk of injury to the vessels supplying the graft. The branch of the left colic artery, whose preservation is crucial to the graft, is located at the left flexure, close to the intestinal wall. 3 Injury to the ascending branch during mobilization of the left flexure will lead to graft necrosis. Because of this risk involved in the standard procedure of left colon interposition, we modified this technique so that preparation of the left colonic flexure is no longer necessary. To construct a colon interposition graft that is long enough, we examined a procedure in which the colon is transected proximally at the site of the cecum and the right colic artery is transected, in addition to ligation of the middle artery.
PATIENTS AND METHODS
Patients
From January 1997 to June 1998, we replaced the esophagus using a colonic interposition in 15 patients (11 men, 4 women, age 57.9 [range 22.8–76.3] years). The indications for surgery were malignant tumors in 14 patients and benign disease in 1 patient. This latter patient had epidermiolysis bullosa, with a widespread detachment of the esophageal mucosa and subsequent covered perforation and formation of strictures. Nine patients (60%) had a squamous cell carcinoma; it was below the tracheal bifurcation in two patients. Seven patients (47%) had an esophageal carcinoma at or above the tracheal bifurcation. In one patient, an additional resection of the hypopharynx had to be performed. Four patients (27%) had Barrett carcinoma. One patient had a cardia carcinoma that infiltrated the esophagus.
Four patients (17.4%) with squamous cell carcinoma received radiochemotherapy before surgery. Radiation was given at 50 Gy. Two patients received only 5-fluorouracil; the other two patients received a combined therapy of 5-fluorouracil and cisplatin. Postoperative UICC classification showed stage I disease (pT1, pN0, pM0) in two patients, stage IIa disease in six patients (pT2-3, pN0, pM0), stage IIb disease in one patient (pT1-2, pN1), and stage III disease in five patients (pT3-4, pN1, pM0).
Before surgery, we examined the complete colon by endoscopy in all patients. Selective angiography of the lower mesenteric artery was not performed routinely. The bowel was prepared by mechanical cleansing. We planned a cervical anastomosis in all patients because of the extent of the benign disease, because of the location of the tumor (at or above the tracheal bifurcation), or because the risk of a thoracotomy was considered too great. Oral antibiotics were administered before surgery and on days 1 through 7 after surgery to decontaminate the colonic interposition graft. 4
Surgical Technique
In all patients, we constructed a long colon graft supplied exclusively by the inferior mesenteric artery. Initially, we mobilized the ascending colon, the right flexure, and the transverse colon. Using transillumination, we then clamped the middle colic artery, the right colic artery, and the connection between the ileocolic and right colic artery temporarily using vascular clamps. In this situation, only the inferior mesenteric artery feeds the ascending and transverse colon. If blood supply remained adequate after prolonged trial clamping, we started constructing the long colonic interposition graft. The right and middle colic arteries were divided and ligated as centrally as possible (Fig. 1). Then we transected the remaining mesentery of the right colon up to the level of the transverse colon. By transecting the ascending colon just above the cecum, we obtained a fairly long and mobile colon graft that could be passed easily up into the thorax and to the cervical region. We did not mobilize the left flexure and the descending colon, and we did not identify the left colic artery and the branching ascending arteries of the left flexure. Distal transection of the colon was not performed at this stage of the procedure.
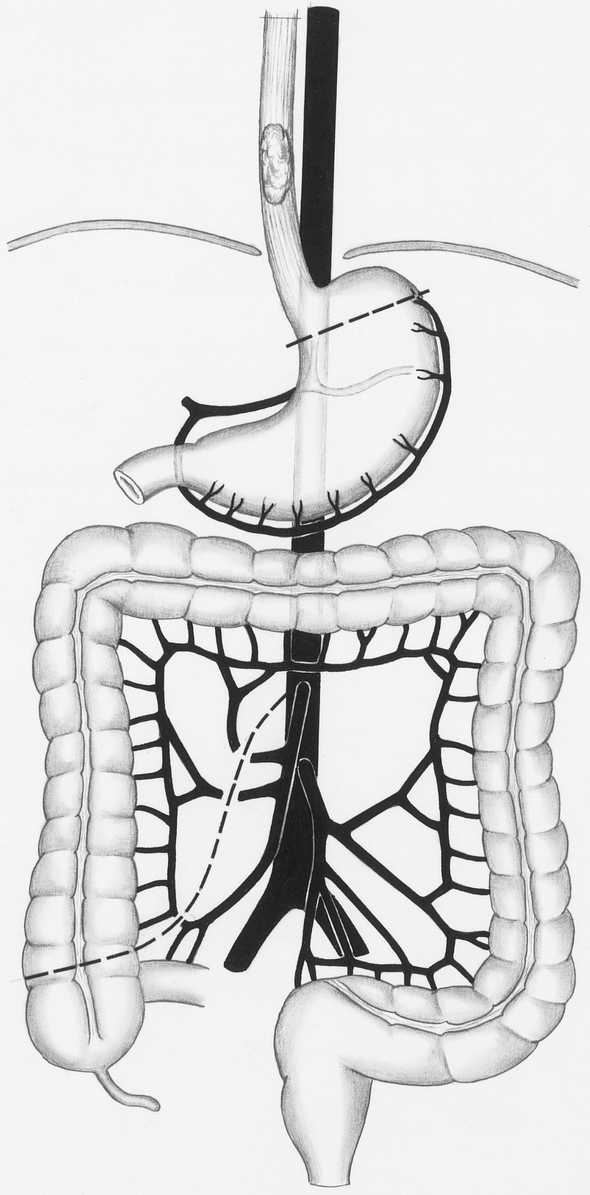
Figure 1. Creation of the colonic interposition graft. After verification of adequate blood supply, the right and middle colic arteries are divided and ligated as centrally as possible. The colon is transected above the cecum.
Then, we removed the esophagus using a conventional transhiatal technique. After widening the hiatus esophageus, we mobilized the distal portion of the esophagus and dissected the lymphatic nodes of the lower mediastinum. In patients with squamous cell carcinoma, we also dissected the lymphatic nodes at the lesser curvature down to the celiac trunk and removed the proximal portion of the stomach, according to the infiltration depth of the tumor. In patients with Barrett carcinoma, we combined an esophagectomy with a gastrectomy and with the corresponding dissection of the epigastric lymphatic nodes. Microscopic perioperative examination of the resection margins was mandatory.
To construct the cervical anastomosis, we approached the esophagus by a left cervical access. During blunt dissection, we mobilized the esophagus by an abdominal access to avoid a thoracotomy. After transection and removal of the esophagus, we brought the colon up to the cervical region. Then we performed the cervical anastomosis using a modified two-layer end-to-end technique (Fig. 2).
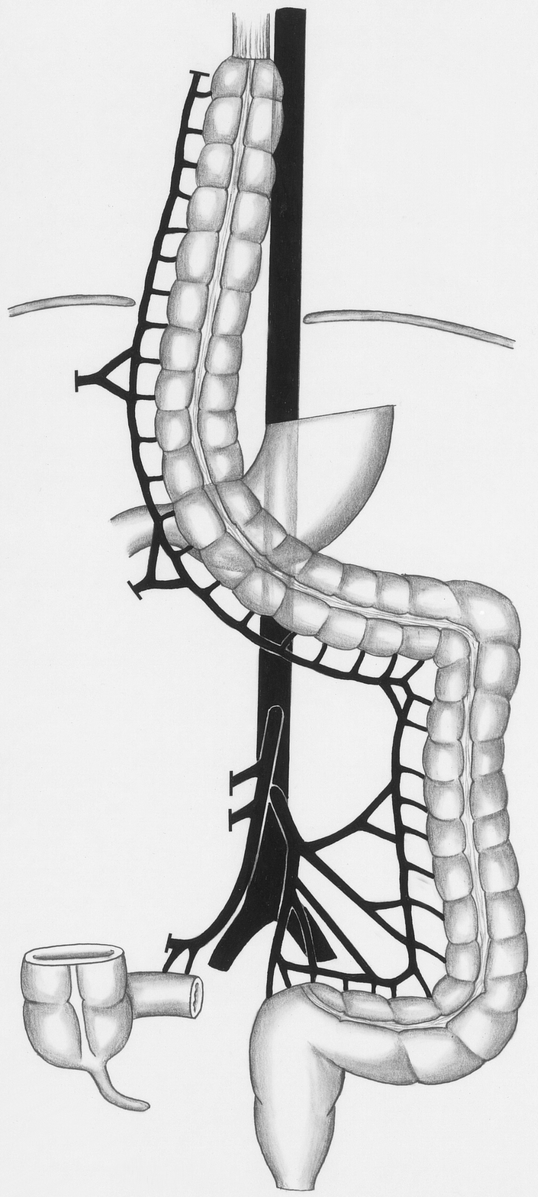
Figure 2. Construction of the cervical anastomosis. After blunt dissection and removal of the esophagus, the colon is pulled up to the cervical region. Due to the length of the graft, mobilization of the left colonic flexure is unnecessary.
After pull-through of the colonic graft, we constructed the anastomosis between the stomach and the colon to reconstruct the passage in the 12 patients in whom the stomach could be preserved. We transected the colon, which descended from inside the thorax into the abdomen, in front of the stomach and constructed an end-to-side anastomosis between the colon and the stomach (Fig. 3). Three patients had a total esophagogastrectomy (esophageal resection and complete removal of the stomach). To reconstruct the intestinal passage in these patients, we performed an anastomosis between the colon graft and a Y-Roux loop of the jejunum. Therefore, we created an isoperistaltic colon interposition graft in all patients. Reconstruction was completed by connecting the cecum to the transverse colon by an end-to-end anastomosis. The expanded colon interposition graft was then attached to the hiatus esophageus using several single-stitch sutures to prevent an elongation of the graft inside the thorax. If present, the remaining stomach was fixed to the diaphragm to avoid passage of the stomach into the thorax.
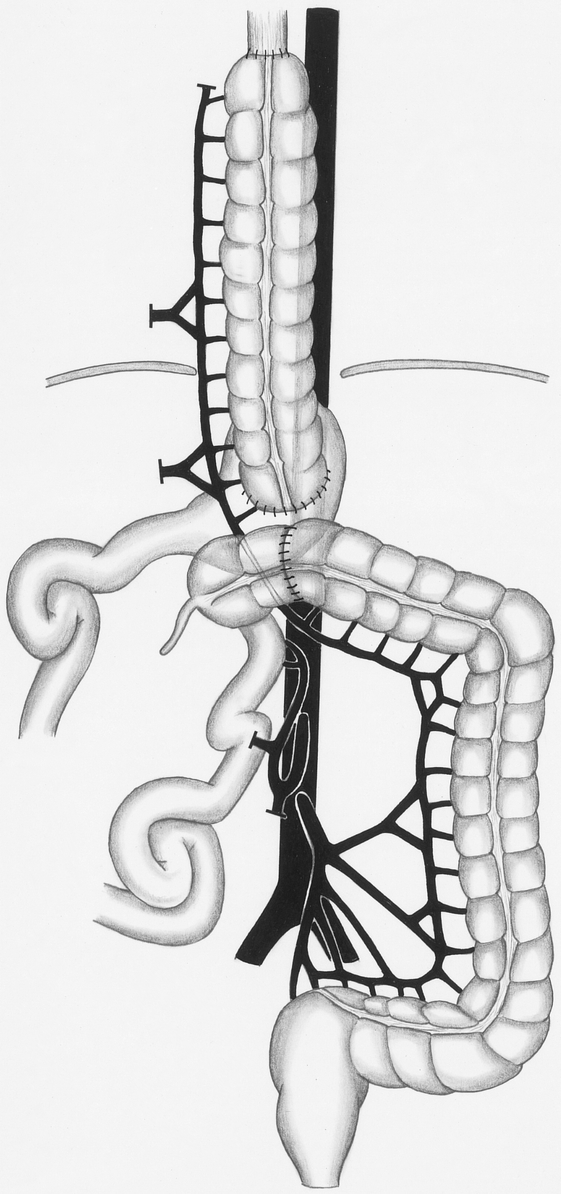
Figure 3. Reconstruction of the intestinal passage. The colon is transected in front of the stomach to construct an end-to-side anastomosis. To complete the reconstruction, the cecum is connected to the transverse colon.
RESULTS
In all patients in whom we planned an esophageal reconstruction with a modified colonic interposition graft, we could create such a graft. In no patient did we have to change the reconstructive procedure because of insufficient intraoperative blood supply. During routine postoperative surveillance (endoscopy), we observed sufficient blood supply to the graft in all patients.
No complications resulted from insufficient blood supply or insufficient venous drainage. Minor complications (pneumonia without respiratory insufficiency) occurred in three patients (20%). In two of these patients, the cause of the pulmonary inflammation was recurrent microaspiration of oral nutrients. Major complications were found four times (27%): one anastomotic leakage, one subcutaneous dehiscence of the abdominal wall, one pulmonary embolism, and one case of pneumonia, which was complicated by a septic state and respiratory insufficiency. The latter patient subsequently died, resulting in a hospital death rate of 7%. The cause of death was herpes pneumonia after preoperative radiochemotherapy. The anastomotic leakage, found in one patient, was evident on the first postoperative day. After surgical reinspection of the anastomosis and suturing of the leak, the further postoperative course was uneventful. Oral nutrient intake was not delayed. One patient, after undergoing esophagectomy and removal of the hypopharynx because of a cervical esophageal carcinoma, could not swallow sufficiently at hospital discharge and had to be fed with a feeding tube.
The average duration of postoperative artificial ventilatory support was 2.8 ± 4.6 (mean ± SD) days (range 0–17 days); eight patients were extubated immediately after surgery. Length of stay on the intensive care unit averaged 6.9 ± 4.5 days (range 1–17 days). The total hospital stay averaged 24.1 ± 15.1 days (range 12–64 days).
DISCUSSION
Almost a century ago, Kelling 5 and Vuillet 6 introduced the use of the colon as an esophageal substitute. Since then, several modifications to this approach have been described, using the left, the right, or the transverse colon as an interposition graft. 1 Interposition of the left colon became the most popular procedure. It requires wide mobilization of the entire colon, ligation of the middle colic artery, and transection of the colon at the right flexure and somewhere between the left flexure and the midportion of the descending colon, depending on the patient’s anatomy. 7
This preference for left colic interposition is based on the vascular anatomy and its natural variation in the colon. According to several autopsy studies, the arterial anastomoses (marginal artery) between the ileocolic and right colic vessels are absent in up to 70% of patients, whereas the collaterals between the left and right colic artery are mostly sufficient. 3,8 Corresponding differences can be found with venous collaterals in the colon. In the left colon, the marginal venous anastomoses are excellent, but ileocolic venous collaterals are insufficient in 20% to 30% of patients. 9,10
Mesenteric angiography does not always confirm these autopsy results. In patients scheduled for colonic interposition, a discontinuity of the superior–inferior mesenteric artery anastomosis at the left flexure was seen in 48%11; discontinuity of the marginal artery between the middle and right colic artery was seen in 70%. 12 However, the relevance of these angiography findings for the selection of the colon graft is questionable: intraoperative trial clamping rarely demonstrates an inadequate collateral flow through arterial anastomoses at the splenic flexure. 11
Clinical results appear to support the superiority of left to right colonic interposition. A combined evaluation of studies that allow a separate analysis of left or right colon grafts revealed a rate of colon necrosis or ischemia of 4.6% (20/438) with use of the left colon and of 10.8% (13/120) with use of the right. 7,11–20
However, even an almost 5% rate of left colonic graft failure cannot be considered optimal, because this complication is potentially life-threatening and adds to the significant general risk of the procedure. A possible reason for ischemic graft failure may be the preparation and mobilization of the left colonic flexure. This step is part of the standard procedure to obtain a left colon interposition graft, but it may damage the ascending branch of the left colic artery or the marginal arteries and veins at this site. To minimize this risk, we modified the conventional technique. If the left flexure is not to be touched, the ascending colon must be included into the graft to obtain sufficient graft length. For this step, the middle and right colic arteries and the collaterals from the ileocolic artery must be ligated. A similar procedure was originally described in two patients by Lees 21 to create a particularly long colon graft. However, a larger series by Osborne et al 22 revealed a graft-related complication rate of 35% when the ascending colon was part of a graft supplied by the left colic artery. In the latter report, the complete colon, including the splenic flexure, was mobilized. Based on our preliminary results, this complication rate appears to be significantly improved if the splenic flexure remains untouched.
Intraoperative temporary clamping demonstrated in each case that arterial blood supply and venous drainage, even of the proximal parts of the colon by the left colic artery and vein, were adequate. This potent collateral circulation allows creation of a long colon graft that primarily includes the ascending and the transverse colon, eliminating the need to mobilize the left flexure to move the graft up to the cervical region. The cervical anastomosis between the esophagus and the colon interposition graft can usually be created easily because there is little tension between the fixed left colonic flexure and the proximal end of the graft. The second advantage of our method is that the distal anastomosis of the graft can be performed later at a variable site and can, therefore, be adjusted exactly to the patient’s anatomy. To complete the reconstruction, we transected the transverse colon at the site where we wanted to perform the anastomosis between the colon and the stomach or small bowel. Finally, the cecum was connected to the transverse colon. It should be noted that the individual anatomy of the colic vasculature may sometimes prevent use of the whole right colon or of its most proximal portion as an interposition graft. Thus, in a few patients, multiple middle colic arteries are present with marginal arteries missing at this site, 3,11 or venous collaterals are absent between the ascending and transverse colon. 9,10 However, such rare anatomic variations should be identified easily during intraoperative preparation and trial clamping.
With our modified technique, the frequency of major complications was 27% and that of anastomotic leakage was 7%. Postoperative graft perfusion was excellent in each patient. The hospital death rate was 7%. Two risk factors may have contributed to the postoperative complications and the one death. Four patients (27%) had received preoperative radiochemotherapy. A recent multivariate analysis revealed such a neoadjuvant therapy to be a separate, significant risk factor with respect to the postoperative course; it was associated with a postoperative death rate of up to 19%. 23 Second, we performed a total esophagogastrectomy in three patients (20%). The combined removal of the esophagus and the complete stomach also carries an increased perioperative and postoperative risk. 24 The significance of these particular risk groups with respect to the perioperative complication rate is also evident from the finding that the one patient who died in our series belonged to one of these risk groups. After preoperative radiochemotherapy, this patient acquired after surgery a rare atypical herpes pneumonia that was difficult to diagnose and treat.
According to the literature, standard colon interposition grafting had a complication rate of 30% to 65% and a death rate of 0% to 23%. However, comparison of our results to those of other studies that used the left colon as an esophageal substitute is limited because of potentially confounding covariables, such as differences in patients, in the type of surgery, or in the time period in which the data were generated. Several reports focus on patients with benign esophageal disorders, 13,14,16,17,20,25 some do not allow a separate analysis of left or right colon grafts, 25–29 and others include patients in whom the esophagus was left in place (retrosternal or subcutaneous bypass) 7,15,18,20,26,29 or who underwent surgery more than two decades ago, when adjuvant and intensive care measures were substantially different. 7,13–17,20,25–28
Despite the above limitations, the results of our series suggest that the described modifications represent an alternative to established procedures for creating a colon interposition graft. Our method may be particularly helpful when a long colonic interposition graft is required, such as after combined esophagectomy and total gastrectomy, or in patients in whom the stomach is no longer available as an esophageal substitute.
Footnotes
Correspondence: Heinrich Fürst, MD, Dept. of Surgery, Klinikum Grosshadern, Marchioninistrasse 15, D-81377 Munich, Germany.
Accepted for publication July 26, 1999.
References
- 1.Rice TW. Colon replacement. In: Pearson FG, Deslauriers J, Ginsberg RJ, et al, eds. Esophageal surgery. New York: Churchill Livingstone; 1995: 761–774.
- 2.Hiebert CA, Bredenberg CE. Selection and placement of conduits. In: Pearson FG, Deslauriers J, Ginsberg RJ, et al, eds. Esophageal surgery. New York: Churchill Livingstone; 1995: 649–656.
- 3.Sonneland J, Anson BJ, Beaton LE. Surgical anatomy of the arterial supply to the colon from the superior mesenteric artery based upon a study of 600 specimen. Surg Gynecol Obstet 1958; 106:385–398. [PubMed] [Google Scholar]
- 4.Schardey HM, Joosten U, Finke U, et al. The prevention of anastomotic leakage after total gastrectomy with local decontamination. Ann Surg 1997; 225:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelling GE. Oesophagoplastik mit Hilfe des Querkolons. Zentralbl Chir 1911; 38:1209–1212. [Google Scholar]
- 6.Vuillet H. De l’oesophagoplastie et des diverses modifications. Semin Med 1911; 31:529–534. [Google Scholar]
- 7.De Meester TR, Johansson KE, Franze J, et al. Indications, surgical technique, and long-term functional results of colon interposition or bypass. Ann Surg 1988; 208:460–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck AR, Baronofsky ID. A study of the left colon as a replacement for the resected esophagus. Surgery 1960; 48:499–509. [PubMed] [Google Scholar]
- 9.Kralik J, Turek K. Die Wichtigkeit des venösen Abflusses aus dem zur Ösophagoplastik verwendeten Kolontransplantat. Zentralbl Chir 1967; 44:2772–2776. [PubMed] [Google Scholar]
- 10.Nicks R. Colonic replacement of the oesophagus. Some observations on infarction and wound leakage. Br J Surg 1967; 54:124–128. [DOI] [PubMed] [Google Scholar]
- 11.Peters JH, Kronson JW, Katz M, DeMeester TR. Arterial anatomic considerations in colon interposition for esophageal replacement. Arch Surg 1995; 130:858–863. [DOI] [PubMed] [Google Scholar]
- 12.Ventemiglia R, Khalil KG, Frazier OH, Mountain CF. The role of preoperative mesenteric arteriography in colon interposition. J Thorac Cardiovasc Surg 1977; 74:98–104. [PubMed] [Google Scholar]
- 13.Belsey R. Reconstruction of the esophagus with left colon. J Thorac Cardiovasc Surg 1965; 49:33–53. [PubMed] [Google Scholar]
- 14.Glasgow JC, Cannon JP, Elkins RC. Colon interposition for benign esophageal disease. Am J Surg 1979; 137:175–179. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins EW, Jr. Long-segment colon substitution for the esophagus. Ann Surg 1980; 192:722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keenan DJM, Hamilton JRL, Gibbons J, Stevenson HM. Surgery for benign esophageal stricture. J Thorac Cardiovasc Surg 1984; 88:182–188. [PubMed] [Google Scholar]
- 17.Curet-Scott MJ, Ferguson MK, Little AG, Skinner DB. Colon interposition for benign esophageal disease. Surgery 1987; 102:568–574. [PubMed] [Google Scholar]
- 18.Cerfolio RJ, Allen MS, Deschamps C, Trtastek VF, Pairolero PC. Esophageal replacement by colon interposition. Ann Thorac Surg 1995; 59:1382–1384. [DOI] [PubMed] [Google Scholar]
- 19.Mansour KA, Bryan FC, Carlson GW. Bowel interposition for esophageal replacement: twenty-five-year experience. Ann Thorac Surg 1997; 64:752–756. [DOI] [PubMed] [Google Scholar]
- 20.Wain JC, Wright CD, Kuo EY, et al. Long-segment colon interposition for acquired esophageal disease. Ann Thorac Surg 1999; 67:313–318. [DOI] [PubMed] [Google Scholar]
- 21.Lees W. Colonic replacement after pharyngolaryngectomy. Br J Surg 1967; 54:541–547. [DOI] [PubMed] [Google Scholar]
- 22.Osborne MP, Griffiths JD, Shaw HJ. Colon transposition in the management of upper gastrointestinal cancer. Cancer 1982; 50:2235–2242. [DOI] [PubMed] [Google Scholar]
- 23.Siewert JR, Bartels H, Bollschweiler E, et al. Plattenepithelcarcinom des Ösophagus. Chirurgie 1992; 63:693–700. [PubMed] [Google Scholar]
- 24.Bartels H, Stein HJ, Siewert JR. Preoperative risk analysis and postoperative mortality of oesophagectomy for resectable oesophageal cancer. Br J Surg 1998; 85:840–848. [DOI] [PubMed] [Google Scholar]
- 25.Buntain WL, Payne WS, Lynn HB. Esophageal reconstruction for benign disease: a long-term appraisal. Am Surg 1980; 46:67–79. [PubMed] [Google Scholar]
- 26.Mansour KA, Hansen HA, Hersh T, Miller JI Jr, Hatcher CR Jr. Colon interposition for advanced nonmalignant esophageal stricture: experience with 40 patients. Ann Thorac Surg 1981; 32:584–591. [DOI] [PubMed] [Google Scholar]
- 27.Cherveniakov A, Cherveniakov P. Colon substitution for radical treatment of cardia and lower third esophageal cancer. Eur J Cardiothorac Surg 1993; 7:601–605. [DOI] [PubMed] [Google Scholar]
- 28.Negre J, Markkula H. Esophagectomy and colon interposition for benign esophageal stricture. Acta Chir Scand 1984; 150:639–642. [PubMed] [Google Scholar]
- 29.Thomas P, Fuentes P, Giudicelli R, Reboud E. Colon interposition for esophageal replacement: current indications and long-term function. Ann Thorac Surg 1997; 64:757–764. [DOI] [PubMed] [Google Scholar]


