Abstract
Objective
To compare the outcome of simultaneous pancreas-kidney transplantation (SPK) and living related donor renal transplantation (LRD) in patients with diabetes.
Summary Background Data
It remains unanswered whether diabetic patients with end-stage renal failure are better served by LRD or SPK.
Methods
Using a longitudinal database, data from all diabetic patients receiving LRD or cadaveric renal transplants or SPKs from January 1986 through January 1996 were analyzed. Patient and graft survival, early graft function, and the cause of patient and graft loss were compared for 43 HLA-identical LRDs, 87 haplotype-identical LRDs, 379 SPKs, and 296 cadaveric renal transplants.
Results
The demographic composition of the SPK and LRD groups were similar, but because of less strict selection criteria in the cadaveric transplant group, patients were 10 years older, more patients received dialysis, and patients had been receiving dialysis longer before transplantation. Patient survival was similar for the SPK and LRD groups but was significantly lower for the cadaveric renal transplant group. Similarly, there was no difference in graft survival between SPK and LRD recipients, but it was significantly lower for recipients in the cadaveric renal transplant group. Delayed graft function was significantly more common in the cadaveric renal transplant group. Discharge creatinine, the strongest predictor of patient and graft survival, was highest in the SPK group and lowest in the HLA-identical LRD group. The rate of rejection within the first year was greatest in SPK patients (77%), intermediate in the haplotype-identical LRD and cadaveric transplant groups (57% and 48%, respectively), and lowest (16%) in the HLA-identical LRD group. Cardiovascular disease was the primary cause of death for all groups. Acute rejection, chronic rejection, and death with a functioning graft were the predominant causes of graft loss.
Conclusions
This study demonstrates that there was no difference in patient or graft survival in diabetic patients receiving LRD or SPK transplants. However, graft and patient survival rates in diabetic recipients of cadaveric renal transplants were significantly lower than in the other groups.
Simultaneous pancreas-kidney transplantation (SPK) is an established treatment for end-stage renal disease resulting from insulin-dependent diabetes mellitus (IDDM). The only consistently successful means of restoring normal glucose homeostasis in patients who cannot produce insulin, it has been performed in more than 8,800 patients worldwide. 1
Diabetic patients with end-stage renal disease currently have three treatment options. First, they can be maintained on supplemental insulin and dialysis. However, with 5-year survival rates of 21%, this is not a favorable approach. 2 Second, they can receive a renal transplant and continue to administer exogenous insulin for glucose control. With 5-year survival rates approaching 70% for diabetic recipients of cadaveric renal transplants and 85% for diabetic recipients of living related (LRD) transplants, patient survival is good with this option. 2,3 Third, both IDDM and end-stage renal disease can be eliminated by SPK. With 5-year survival rates approaching 85%, it is also an excellent option in selected patients. 1
Although it was hypothesized that good glucose control might decrease the long-term complications of IDDM, this was not conclusively demonstrated until 1993, when the Diabetes Control and Complications Trial study showed that tight glucose control significantly decreased nephropathy, retinopathy, and neuropathy. 4 This benefit was achieved at a cost, however: the tightly controlled patients suffered a two- to threefold increase in episodes of severe hypoglycemia. Pancreas transplantation, which reestablishes normal glucose homeostasis, is therefore an optimal treatment for IDDM. However, SPK is also not without costs. Not only is it more expensive than kidney transplantation, but it also imposes greater surgical and immunologic risks. 5–7 Also, by requiring transplantation of the associated cadaveric kidney, it can potentially preempt the use of an LRD kidney. Thus, it is important to determine whether SPK is more beneficial than, or at least as good as, kidney transplantation alone.
Most patients at our center have the option of choosing a cadaveric renal transplant, an LRD (or a living unrelated donor transplant), or an SPK. Those at increased surgical risk, however, are offered only cadaveric renal transplantation. In this study, we compared the outcomes of LRD and SPK. To provide additional clinical perspective, we also analyzed the outcome of cadaveric renal transplantation in diabetic patients.
PATIENTS AND METHODS
Patients
Our transplant center maintains a longitudinal database on all transplant recipients. Data from all diabetic patients receiving cadaveric renal transplants, LRDs, or SPKs at our center from January 1986 through January 1996 were analyzed. This included a total of 43 HLA-identical LRDs, 87 haplotype-identical LRDs, 379 SPKs, and 296 cadaveric renal transplants.
Preservation
Nearly all cadaveric kidneys, both those for cadaveric and SPK transplantation, were perfused with 4°C oxygenated UW solution on a Belzer pulsatile perfusion apparatus. All LRD kidneys were flushed with iced lactated Ringer’s solution (supplemented with 22.3 mEq bicarbonate, 10,000 U heparin, 25 g mannitol, and 5 mL 1% procaine) and transplanted immediately after removal from the donor.
Major Histocompatibility Class (MHC) Matching
Patients receiving cadaveric and LRD renal allografts were prospectively cross-matched and optimized with respect to HLA A, B, and DR matching. SPK recipients were matched with respect to ABO antigens; although screened for antidonor antibodies, they were not matched with respect to MHC antigens.
Immunosuppression
Patients receiving SPK allografts received induction immunosuppressive therapy consisting of an antilymphocyte antibody preparation (OKT3 5 mg/day or ATGAM 15 mg/kg/day), an antimetabolite (azathioprine 1 mg/kg/day or mycophenolate mofetil [MMF] 1,500 mg twice daily), and a tapered bolus of methylprednisolone, beginning with 1 g rapidly tapered to 30 mg/day prednisone. Patients receiving SPK allografts were maintained on a regimen including a calcineurin inhibitor (cyclosporin A or, occasionally, tacrolimus [FK 506]), azathioprine or MMF, and prednisone. Patients receiving cadaveric renal allografts received the same induction and maintenance regimen as SPK recipients, except for lower doses of MMF (1,000 mg twice daily) and prednisone (starting with 500 mg/day). Most patients receiving haplotype-identical LRD allografts also received induction antilymphocyte therapy (except for early in the series, when they did not). Patients receiving HLA-identical LRD renal allografts received no antilymphocyte induction therapy and, unlike the other groups, were rapidly tapered off prednisone, taking only azathioprine and cyclosporin A (or FK 506). With a target maintenance level for cyclosporin A of 300 to 400 mg/dL, cyclosporin A levels were maintained approximately 100 mg/dL higher in the SPK patients than in the renal transplant patients.
Statistical Analysis
Patient and allograft survival rates were determined using the Kaplan-Meier product limit method and compared using the log rank test. Discrete rates were compared using Fisher’s exact test or chi square analysis. Discrete variables were compared using rank analysis of variance or, for pair-wise comparisons, Fisher’s protected least significant difference procedure applied to ranked discrete variables. Multivariate analysis was performed using the Cox proportional hazards model. P ≤ .05 was considered statistically significant and all P values reflected the results of two-tailed analyses. All statistical analyses were performed using the SAS system (SAS Institute, Cary, NC).
RESULTS
Patient Demographics
As a group (Table 1), patients receiving cadaveric renal allografts were approximately 10 years older at the time of transplantation. The sexual makeup of all four groups was similar. The duration of IDDM before transplantation did not differ between groups, but the percentage of patients receiving dialysis before transplantation was greater in the cadaveric transplant group. Similarly, the length of dialysis before transplantation was significantly longer in the cadaveric transplant group.
Table 1. PATIENT DEMOGRAPHICS
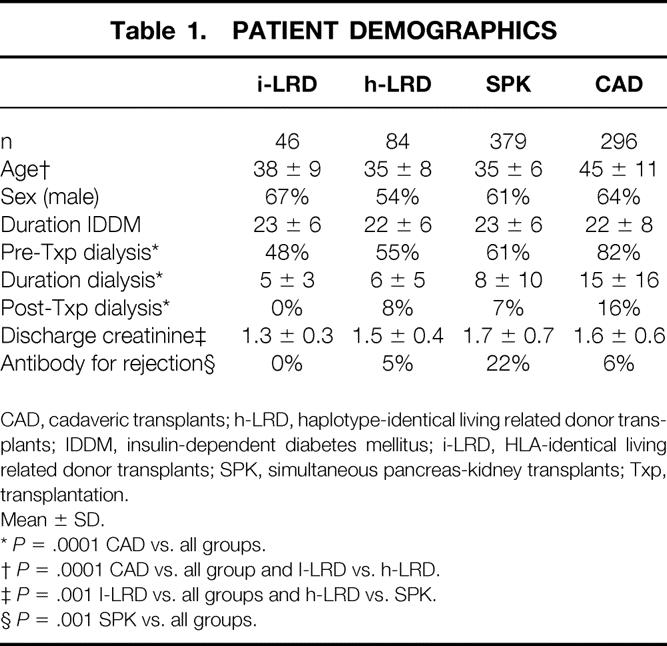
CAD, cadaveric transplants; h-LRD, haplotype-identical living related donor transplants; IDDM, insulin-dependent diabetes mellitus; i-LRD, HLA-identical living related donor transplants; SPK, simultaneous pancreas-kidney transplants; Txp, transplantation.
Mean ± SD.
*P = .0001 CAD vs. all groups.
†P = .0001 CAD vs. all group and I-LRD vs. h-LRD.
‡P = .001 I-LRD vs. all groups and h-LRD vs. SPK.
§P = .001 SPK vs. all groups.
MHC Characteristics
The distribution of MHC matching followed what would be expected on the basis of pretransplantation matching protocols. The SPK group, with an average of 4.2 MHC mismatches (the mean for random pairing at the A, B, and DR loci is 4.5 mismatches 6), had the greatest number of single and double mismatches at the A, B, and DR loci (P < .0001). The cadaveric transplant group, with an average of 3.5 mismatches, also had a fairly high mismatch rate, but because of the optimization of MHC compatibility (zero mismatch kidneys), they had significantly fewer MHC mismatches than the SPK group. Of course, there were no mismatches in the HLA-identical LRD group and no double mismatches in the haplotype-identical LRD group, which on average had 2.3 mismatches.
Patient Survival
As shown in Figure 1, Kaplan-Meier patient survival did not differ between the HLA-identical LRD, haplotype-identical LRD, and SPK groups, but it was significantly lower in the cadaveric transplant group. At 1 year, patient survival rates in the HLA-identical LRD, haplotype-identical LRD, SPK, and cadaveric transplant groups were 100%, 99%, 96%, and 94%, respectively. At 5 years, patient survival rates decreased to 94%, 85%, 88%, and 72%, respectively.

Figure 1. Patient survival (Kaplan-Meier estimates). Overall:P = .0001. Pairwise: cadaveric transplants (CAD) versus simultaneous pancreas-kidney transplants, P = .0001; CAD versus haplotype-identical living related donor transplants, P = .02; CAD versus HLA-identical living related donor transplants, P = .01;P = NS for all other combinations.
Graft Survival
Renal allograft survival (Fig. 2) did not differ significantly between the HLA-identical LRD, haplotype-identical LRD, or SPK groups, but it was significantly lower in the cadaveric transplant group. At 1 year, renal allograft survival rates in the HLA-identical LRD, haplotype-identical LRD, SPK, and cadaveric transplant groups were 96%, 94%, 87%, and 86%, respectively. At 5 years, renal allograft survival rates decreased to 85%, 72%, 78%, and 64%, respectively.
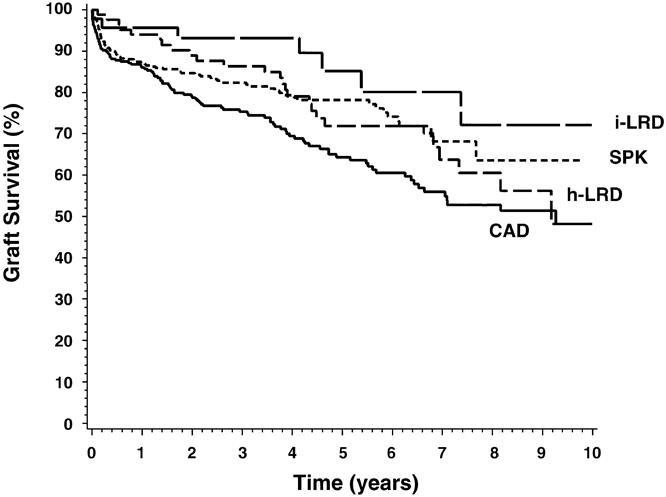
Figure 2. Renal allograft survival (Kaplan-Meier estimates). Overall:P = .006. Pairwise: cadaveric transplants versus simultaneous pancreas-kidney transplants, P = .005; cadaveric transplants versus HLA-identical living related donor transplants, P = .009;P = NS for all other combinations.
Early Graft Function
Two variables were used to characterize renal allograft function immediately after transplantation: the rate of dialysis immediately after the transplant and the serum creatinine level at discharge.
As shown in Table 1, HLA-identical LRD kidneys performed best, with no patients requiring dialysis after transplantation. Interestingly, despite the longer preservation times encountered in the SPK group, early allograft performance, as indicated by the requirement for dialysis after transplantation, did not differ between the haplotype-identical LRD and SPK groups. The cause of early graft dysfunction, however, did differ between the two groups. All early graft dysfunctions in the haplotype-identical LRD group were due to rejection, whereas only 33% of early graft dysfunction in the SPK group was due to acute rejection. Instead, acute tubular necrosis was the primary cause, accounting for 41% of cases of delayed graft function in the SPK group. The cadaveric transplant group had the worst initial allograft performance, with 16% of patients requiring dialysis after transplantation (P = .0001), primarily due to acute tubular necrosis (76%).
Discharge creatinine, which may reflect the state of the kidney before procurement, preservation injury, rejection, drug toxicity, or the level of cyclosporin A or tacrolimus, varied significantly between groups (P = .0001). As shown in Table 1, the SPK and cadaveric transplant groups had the highest average discharge creatinine levels. The haplotype-identical LRD group, with minimal preservation injury but relatively frequent early rejection, had an intermediate level; the HLA-identical LRD group, which had minimal rejection and minimal preservation injury, had the lowest mean discharge creatinine. On multivariate analysis, the discharge creatinine was found to be the best predictor of both patient and renal allograft loss. Indeed, for each 1-mg/dL increase in creatinine above the mean for the HLA-identical LRD group (the index group), the risk ratios for patient and graft survival increased by 1.5 (P = .007) and 1.8 (P = .0001), respectively. In fact, when corrected for discharge creatinine, group designation had no significant bearing on patient or graft survival, even for the cadaveric transplant group. Thus, it appeared that long-term graft and patient survival rates were largely determined at the outset.
Rejection
As shown in Figure 3, rates of rejection (diagnosed on the basis of renal biopsy) differed significantly between groups. Rejection occurred within the first year in only 16% of patients receiving an HLA-identical LRD renal transplant. The cadaveric transplant group, with a 1-year rate of 48%, had the next lowest rate of rejection, lower even than that of the haplotype-identical LRD group, which had a rate of 57%. Rejection occurred most commonly in the SPK patients: 77% had at least one episode of rejection in the first year. The use of anti-T-cell antibody preparations to treat refractory rejection reflected the rates of rejection overall, with a significantly larger requirement for the SPK patients (Table 1).
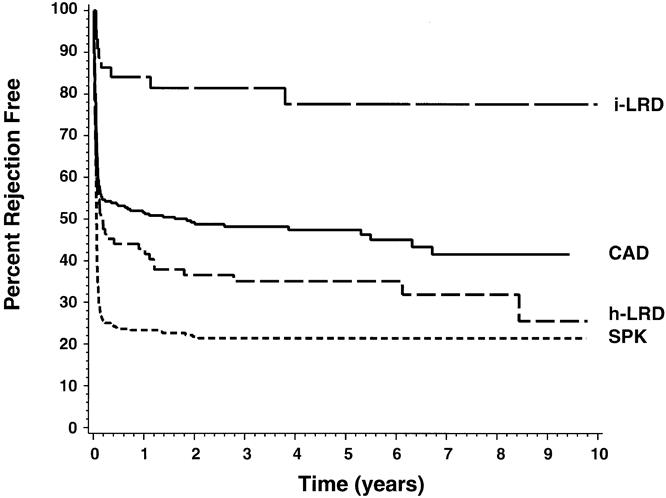
Figure 3. Rates of renal allograft rejection (Kaplan-Meier estimates). Overall:P = .0001. Pairwise:P = .0001 for all comparisons except cadaveric transplants versus haplotype-identical living related donor transplants, where P = .05.
Cause of Death
As shown in Table 2, cardiovascular disease was the most common cause of death in all four groups. Cardiac and cerebrovascular disease accounted for 36% of deaths in SPK patients, 50% of deaths in cadaveric transplant patients, 59% of deaths in haplotype-identical LRD patients, and 100% of deaths in the HLA-identical LRD patients. In the SPK and cadaveric transplant patients, who received more intense immunosuppression, infection and malignancy also were significant causes of death.
Table 2. CAUSE OF DEATH AND GRAFT LOSS (%)

Percentages were calculated with respect to the total population of each group.
CAD, cadaveric transplants; h-LRD, haplotype-identical living related donor transplants; i-LRD, HLA-identical living related donor transplants; SPK, simultaneous pancreas-kidney transplants.
Cause of Graft Loss
As shown in Table 2, death with a functioning graft was a major cause of graft loss in all four groups. It was the dominant cause in HLA-identical LRD and cadaveric transplant recipients, accounting for 59% and 46% of graft losses, respectively. Acute rejection was also important, especially for the SPK group, where it was responsible for 36% of total graft losses. Chronic rejection also contributed significantly to graft loss, especially in the haplotype-identical LRD patients, where it caused 54% of total graft losses. Recurrent disease was not an important cause of graft loss.
DISCUSSION
Diabetes mellitus is the most common endocrine disease worldwide and is the leading chronic disease in children. Despite the success of exogenous insulin therapy, numerous long-term sequelae develop in patients with diabetes, including end-stage renal failure, cardiovascular disease, autonomic and somatic neuropathy, and blindness. Chronically abnormal lipid metabolism, accelerated atherosclerosis, and destruction of the microvascular system result in global vascular disease, leading to amputations and premature death from myocardial infarctions and cerebrovascular accidents. Occurring in approximately 1% of the population, diabetes accounts for more than 160,000 deaths annually in the United States. 7
The impetus for SPK transplantation is to produce a lasting euglycemic state that enhances quality of life and prevents, arrests, or perhaps even reverses the otherwise inexorable progression of the destructive effects of diabetes. The resumption of normal glucose homeostasis through SPK transplantation provides several benefits. First, and perhaps most importantly, quality of life is improved. 8–12 Second, as demonstrated by the Minnesota group and others, the recurrence of diabetic nephropathy is attenuated. 13–16 Third, SPK transplantation reduces diabetic retinopathy. 17 Fourth, the progression of diabetic neuropathy may be halted and in some cases reversed. 18–21 This includes improvements in autonomic neuropathy, enhancing both cardiac reflex function and gastric motility. 21 Even diabetic vesicopathy has been shown to improve after SPK transplantation. 22 Lastly, diabetic cardiovascular disease is attenuated after SPK transplantation. 23–26 This wide array of healing that occurs after pancreas transplantation could tip the scales away from LRD renal transplantation toward SPK transplantation, but only as long as the basic requirement that the two are at least equivalent in patient and graft survival is met.
Because living related kidney allografts have less immunologic disparity and comparatively minimal preservation injury, their graft survival significantly exceeds that of cadaveric renal transplants. 3 However, in the setting of diabetes, with the possibility of recurrent diabetic nephropathy and other disabling complications, the addition of a pancreas transplant might provide benefits that outweigh the advantages of LRD renal transplantation. Indeed, with 1- and 5-year pancreas survival rates of 86% and 74%, pancreas allograft survival in this series was quite good. Still, the possible benefit to the patient must be weighed against the potential loss of an opportunity to use an LRD kidney.
In this study, we compared the outcome of SPK transplantation and LRD transplantation and found that patient and renal allograft survival did not differ significantly between the two. Even when the immunologically optimal HLA-identical LRD kidneys were compared with SPK renal transplants, no significant difference in patient or graft survival was detected. Thus, on the basis of this survival criterion alone, there is no compelling reason to choose one over the other. However, the magnitudes of the differences between groups, although not significant, were perhaps large enough to be considered important from a clinical point of view (invoking inadequate statistical power). Specifically, with 5-year patient and graft survival rates of 95% and 84%, the HLA-identical LRD group survival rates were 7% and 6% higher than those of the SPK group. This difference was substantial only for the HLA-identical LRD group; there was no real difference in 5-year patient and graft survival rates between the haplotype-identical LRD and SPK patients.
To shed further light on the comparisons of survival between LRD and SPK renal allografts in diabetic patients, early graft function was analyzed. Discharge creatinine, the strongest predictor of graft and patient survival, was significantly greater in the SPK group than in both LRD groups, indicating that the LRD groups had better early allograft performance. SPK patients received higher target levels of cyclosporin A, however, which may partly explain the difference. Still, the SPK group also had a higher rate of dialysis after transplantation. Thus, considering both survival data and early graft function, it may be safest to conclude that HLA-identical LRD transplants have some survival advantage. However, the differences are small and should be weighed against the possible benefits of pancreas transplantation.
Future studies may clarify the issue of SPK versus LRD transplantation in diabetic patients. Not only will they have the benefit of larger groups, but with the current improvements in surgical technique and immunosuppression (e.g., MMF has reduced the rate of rejection by >50% in our SPK transplant population), early injury to SPK transplants will continue to become less frequent and less severe, allowing SPK survival to approach that of HLA-identical LRD transplants.
Although not directly pertinent to the comparison of outcome between diabetic recipients of LRD and SPK renal transplants, to provide additional clinical perspective, we also analyzed patient and graft survival in cadaveric renal transplants in diabetic patients. Although they had significantly less rejection than the SPK patients, both patient and graft survival rates were significantly lower in the cadaveric transplant group. Interestingly, when graft and patient survival rates were analyzed after correcting for discharge creatinine, the differences in survival between groups, including the cadaveric transplant group, disappeared, indicating that early graft function, more than type of transplant, determined long-term survival.
Strong selection biases shaped the cadaveric transplant population, differentiating them from the SPK and LRD groups. Older patients and patients with advanced cardiovascular disease were considered only for cadaveric transplantation. Consequently, the patients in the cadaveric transplant group were older and more likely to have received dialysis before transplantation; accordingly, they had the highest rates of death from cardiovascular disease and death with a functioning graft.
The cadaveric transplant group was also affected by donor selection biases. Acceptance criteria was less stringent for cadaveric kidneys than for LRD and SPK kidneys; marginal donors were not used for LRD or SPK transplantation. Therefore, acute tubular necrosis was the predominant cause of early graft dysfunction in the cadaveric transplant patients, and their requirement for dialysis after transplantation was greatest.
Using the entire UNOS database, patient and graft survival in diabetic patients receiving cadaveric renal allografts has been compared with that of SPK patients. 3 Consistent with the present findings, the 5-year survival rate for SPK patients, 81%, was significantly greater than the 70% survival rate observed in diabetic recipients of cadaveric kidneys alone. Similarly, the 5-year graft survival rate was significantly better in SPK patients (67% vs. 55%). Notably, rates of rejection and delayed graft function were similar to those of the present study. Several other comparisons of SPK and cadaveric renal transplantation in diabetic patients have been published. Cheung et al 27 from Minnesota showed that for patients younger than 45 years, there was no difference in 2-year patient or graft survival rates, but older patients receiving cadaveric kidneys alone fared significantly better than older SPK recipients. The Iowa group, who analyzed a cohort of patients younger than 45 years, showed no difference in long-term survival between cadaveric and SPK recipients. 28 Both studies, showing good results in young cadaveric transplant recipients, lend strength to the hypothesis that the worse outcome of the cadaveric transplant group in the present study is at least partly a patient selection effect. Nonetheless, consistent with the present findings, both Stratta et al 29 and Schulak et al 30 have demonstrated that SPK patients have better long-term rates of patient and graft survival than those receiving cadaveric transplants.
Because there are few data in diabetic patients on long-term outcome based on pretransplantation cardiovascular status, it would have been ideal to have quantified the extent of pretransplantation coronary artery disease in the four patient groups. Unfortunately, our pretransplantation cardiac data were incomplete. Further, the extent of the cardiac evaluations and the methods used for risk assessment varied widely. Thus, no meaningful comparison could be performed.
It is interesting that the cadaveric renal transplant patients suffered less rejection than both the haplotype-identical LRD and the SPK groups. The fact that SPK patients suffer rejection more frequently than cadaveric transplant recipients has been shown previously, 3 but it was unexpected that, with their relatively greater MHC compatibility, the rate of rejection in the haplotype-identical LRD group would be significantly greater than that of the cadaveric transplant patients and that the haplotype-identical LRD group would have had the highest rates of acute tubular necrosis attributable to rejection. This may reflect the fact that early in the series, a fraction of the haplotype-identical LRD patients did not receive induction antibody therapy. Interestingly, the haplotype-identical LRD group also had the highest rate of graft loss due to chronic rejection, perhaps hinting that the anti-T-cell antibody used to induce the cadaveric transplant and SPK patients may have provided some protection against chronic injury.
Immunologic graft loss was not common and reflected the rates of rejection overall. As would be expected on the basis on their greater MHC disparity, the SPK and cadaveric transplant groups, with rates of 8% and 7% respectively, had the most graft loss due to acute rejection. The haplotype-identical LRD group, which as discussed above had an unusually high rate of graft loss due to chronic rejection, lost only 4% of grafts to acute rejection. Patients who received HLA-identical LRD kidneys had minimal graft loss due to acute rejection.
Graft loss due to death with a functioning graft was common in all groups and was the predominant cause of graft loss for both HLA-identical LRD and cadaveric transplant recipients. As would be expected for diabetic transplant patients, cardiovascular disease was the predominant cause of death. Thus, these patients must be followed up vigilantly for progression of cardiovascular disease. We recommend cardiac stress testing every 3 years after the transplant and liberal use of carotid duplex ultrasound studies.
In summary, this study has demonstrated that there was no statistically significant difference in patient or graft survival rates between diabetic recipients of LRD transplants and SPK transplants. The finding that recipients of HLA-identical LRD transplants had better early graft function, however, may indicate that this group actually had some small survival advantage. However, with the recent improvements in technique and in immunosuppression for SPK transplants, these differences may disappear in subsequent analyses. Graft and patient survival rates in diabetic recipients of cadaveric kidneys alone, however, were significantly lower than in the other groups, perhaps due in part to patient and donor selection effects.
Acknowledgements
The authors thank Barbara Voss for assistance with the database and Janet Fox and Naomi Erickson for preparation of the manuscript.
Footnotes
Correspondence: Hans W. Sollinger, MD, PhD, Dept. of Surgery, University of Wisconsin, 600 Highland Ave., Room H4/780, Madison, WI 53792-7375.
Dr. Rayhill is currently with the Section of Organ Transplantation, Department of Surgery, University of Iowa, Iowa City, IA.
Dr. Kirk is currently with the Navy/NIDDK Transplantation and Autoimmunity Branch, National Institutes of Health, Bethesda, MD.
Dr. Van der Werf is currently with the Department of Surgery, University of Florida, Gainesville, FL.
Accepted for publication June 16, 1999.
References
- 1.Gruessner A, Sutherland DER. Pancreas transplantation in the United States and non-US as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). In: Cecka JM, Terasaki PI, eds. Clinical Transplants 1996. Los Angeles: UCLA Tissue Typing Laboratory; 1997: 47–67. [PubMed]
- 2.USRDS 1998 Annual Data Report. Reference tables E 50, 58, 66, 74, 82, 90. USRDS, April 1998.
- 3.Cecka JM. The UNOS Scientific Renal Transplant Registry. In: Cecka MJ, Terasaki PI, eds. Clinical Transplants 1996. Los Angeles: UCLA Tissue Typing Laboratory; 1997: 1–14. [PubMed]
- 4.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986. [DOI] [PubMed] [Google Scholar]
- 5.Rosen CB, Frohnert PP, Velosa JA, Engen DE, Sterioff S. Morbidity of pancreas transplantation during cadaveric renal transplantation. Transplantation 1991; 51:123–127. [DOI] [PubMed] [Google Scholar]
- 6.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med 1995; 333:333–336. [DOI] [PubMed] [Google Scholar]
- 7.Pirsch JD, Andrews C, Hricik DE, et al. Pancreas transplantation for diabetes mellitus. Am J Kid Dis 1996; 27:444–450. [DOI] [PubMed] [Google Scholar]
- 8.Adang EMM, Engel GL, van Hooff JP, Kootstra G. Comparison before and after transplantation of pancreas-kidney and pancreas-kidney with loss of pancreas. A prospective controlled quality of life study. Transplantation 1996; 62:754–758. [DOI] [PubMed] [Google Scholar]
- 9.Corry RJ, Zehr P. Quality of life in diabetic recipients of kidney transplants is better with the addition of the pancreas. Clin Transplantation 1990; 4:238–241. [PubMed] [Google Scholar]
- 10.Kiebert GM, van Oosterhout ECAA, van Bronswijk H, Lemkes HHPJ, Gooszen HG. Quality of life after combined kidney-pancreas or kidney transplantation in diabetic patients with end-stage renal disease. Clin Transplant 1994; 8:239–245. [PubMed] [Google Scholar]
- 11.Esmatjes E, Ricart MJ, Fernandez-Cruz L, Gonzalez-Clemente JM, Saenz A, Astudillo E. Quality of life after successful pancreas-kidney transplantation. Clin Transplant 1994; 8:75–78. [PubMed] [Google Scholar]
- 12.Nathan DM, Fogel H, Norman D, et al. Long-term metabolic and quality of life results with pancreatic/renal transplantation in insulin-dependent diabetes mellitus. Transplantation 1991; 52:85–91. [DOI] [PubMed] [Google Scholar]
- 13.Bilous RW, Mauer SM, Sutherland DER, Najarian JS, Goetz FC, Steffes MW. The effects of pancreatic transplantation on the glomerular structure of renal allografts in patients with insulin-dependent diabetes. N Engl J Med 1989; 321:80–85. [DOI] [PubMed] [Google Scholar]
- 14.Barbosa J, Steffes MW, Sutherland DER, Connett JE, Rao KV, Mauer SM. Effect of glycemic control on early diabetic renal lesions. JAMA 1994; 272:600–606. [PubMed] [Google Scholar]
- 15.Wilczek HE, Jaremko G, Tyden G, Groth CG. Evolution of diabetic nephropathy in kidney grafts. Transplantation 1995; 59:51–57. [DOI] [PubMed] [Google Scholar]
- 16.El-Gebely S, Hathaway DK, Elmer DS, Gaber LW, Acchiardo S, Gaber AO. An analysis of renal function in pancreas-kidney and diabetic kidney-alone recipients at two years following transplantation. Transplantation 1995; 59:1410–1415. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Klein R, Moss SE, et al. The influence of combined kidney-pancreas transplantation on the progression of diabetic retinopathy. Ophthalmology 1994; 101:1071–1076. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy WR, Navarro X, Goetz FC, Sutherland DER, Najarian JS. Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med 1990; 322:1031–1037. [DOI] [PubMed] [Google Scholar]
- 19.van Oosterhout ECAA, van Dijk JG, Gooszen HG, van der Woude FJ, Lemkes HHPJ. Nerve conduction velocity after combined renal-pancreas transplantation. Transpl Proc 1994; 26:496. [PubMed] [Google Scholar]
- 20.Gaber AO, Hathaway DK, Abell T, Cardoso S, Hartwig MS, El Gebely S. Improved autonomic and gastric function in pancreas-kidney vs. kidney alone transplantation contributes to quality of life. Transpl Proc 1994; 26:515–516. [PubMed] [Google Scholar]
- 21.Laftavi MRA, Chapuis F, Rahbar M, et al. Diabetic polyneuropathy outcome after successful pancreas transplantation: 1- to 9-year follow-up. Transpl Proc 1995; 27:1406–1409. [PubMed] [Google Scholar]
- 22.Martin X. Improvement of diabetic vesicopathy after pancreatic transplantation. Transpl Proc 1995; 27:2441–2443. [PubMed] [Google Scholar]
- 23.Cheung ATW, Perez RV, Basadonna GP, Cox KL, Bry WI. Microangiopathy reversal in successful simultaneous pancreas-kidney transplantation. Transpl Proc 1994; 26:493–495. [PubMed] [Google Scholar]
- 24.Abendroth D, Landgraf R, Pfeiffer M, Reininger J, Seidel D, Land W. Diabetic microangiopathy and blood viscosity changes after pancreas and kidney transplantation. Transpl Proc 1994; 26:491–492. [PubMed] [Google Scholar]
- 25.Gaber AO, El-Gebely S, Sugathan P, et al. Changes in cardiac function of type I diabetics following pancreas-kidney and kidney-alone transplantation. Transpl Proc 1995; 27:1322–1323. [PubMed] [Google Scholar]
- 26.Konigsrainer A, Foger BH, Miesenbock G, Patsch JR, Margreiter R. Pancreas transplantation with systemic endocrine drainage leads to improvement in lipid metabolism. Transpl Proc 1994; 26:501–502. [PubMed] [Google Scholar]
- 27.Cheung AHS, Sutherland DER, Gillingham KJ, et al. Simultaneous pancreas-kidney transplant versus kidney transplant alone in diabetic patients. Kidney Int 1992; 41:924–929. [DOI] [PubMed] [Google Scholar]
- 28.Douzdjian V, Abecassis MM, Corry RJ, Hunsicker LG. Simultaneous pancreas-kidney versus kidney-alone transplants in diabetics: increased risk of early cardiac death and acute rejection following pancreas transplants. Clin Transpl 1994; 8:246–251. [PubMed] [Google Scholar]
- 29.Stratta RJ, Taylor RJ, Ozaki CF, et al. The analysis of benefit and risk of combined pancreatic and renal transplantation versus renal transplantation alone. Surg Gynecol Obstet 1993; 177:163–171. [PubMed] [Google Scholar]
- 30.Schulak JA, Mayes JT, Hricik DE. Kidney transplantation in diabetic patients undergoing combined kidney-pancreas or kidney-only transplantation. Transplantation 1992; 53:685–687. [PubMed] [Google Scholar]


