Abstract
Objective
To report the authors’ experience in extensive abdominal surgery after caustic ingestion, and to clarify its indications.
Summary Background Data
After caustic ingestion, extension of corrosive injuries beyond the esophagus and stomach to the duodenum, jejunum, or adjacent abdominal organs is an uncommon but severe complication. The limit to which resection of the damaged organs can be reasonably performed is not clearly defined.
Methods
From 1988 to 1997, nine patients underwent esophagogastrectomy extended to the colon (n = 2), the small bowel (n = 2), the duodenopancreas (n = 4), the tail of the pancreas (n = 1), or the spleen (n = 1). Outcome was evaluated in terms of complications, death, and function after esophageal reconstruction.
Results
Five patients required reintervention in the postoperative period for extension of the caustic lesions. There were two postoperative deaths. Seven patients had secondary esophageal reconstruction 4 to 8 months (median 6 months) after initial resection. Three additional patients died 8, 24, and 32 months after the initial resection. Three survivors eat normally, and one has unexplained dysphagia.
Conclusions
An aggressive surgical approach allows successful initial treatment of extended caustic injuries. Early surgical treatment is essential to improve the prognosis in these patients.
The surgical treatment of severe caustic injuries limited to the esophagus and stomach has been described extensively. 1 However, situations in which corrosive lesions extend beyond the stomach and the esophagus to the duodenum, the jejunum, or the adjacent thoracoabdominal organs have been seldom reported. They are related to massive ingestion of strong caustics or to delayed surgical management. Complication and death rates in such patients are high and depend on the type and extent of resection. 2–6 The limit to which resection of the damaged organs can be reasonably performed is not clearly defined. The aim of this study is to report our results after extended total esophagogastrectomy for caustic ingestion in nine consecutive patients to clarify the indications for resection and the distinctive features of reconstructive surgery in these patients.
PATIENTS AND METHODS
From January 1988 to December 1997, six women and three men with a mean age of 45.8 years (range 36–62) underwent total esophagogastrectomy extended to other abdominal organs after oral absorption of corrosive substances for attempted suicide. Six were referred from another institution; three were admitted directly to our digestive surgery unit. One patient also had a cervical wound caused by an electric knife, which required emergency tracheotomy. Another had a vertebral fracture with neurologic symptoms after defenestration. In six patients, the causal substance was caustic soda-based strong alkali. The other patients had ingested a solution of chlorine, ammonia, and hydrochloric acid, respectively. The absorbed volumes ranged from 100 to 250 mL.
On admission to the intensive care unit, each patient underwent blood gas analysis, chest x-ray, upper digestive and tracheobronchial endoscopy, and ear, nose, and throat examination. Investigation of adjacent abdominal organs was performed during laparotomy.
Initial investigations showed major metabolic disorders in four patients. Two had septic shock. Upper digestive endoscopy revealed extended esophagogastric necrosis in all patients. The duodenum could be examined in five patients and exhibited necrosis (n = 2), ulcerations (n = 2), or erythema (n = 1). Tracheobronchial endoscopy revealed posterior necrosis of the trachea and left hilar bronchus in one patient. The mean interval between caustic ingestion and surgery was 30 hours (range 6–120).
RESULTS
Surgical Findings
Four patients had peritonitis after gastric perforation in three and colonic perforation in one. One patient had mucosal necrosis of the duodenum. Five patients had transmural duodenal necrosis; in one patient it was limited to the first duodenum and in the other four it extended to the entire duodenum, involving 40 cm of jejunum in one of them. Pancreatic head necrosis was seen in one of these patients. Isolated jejunal necrosis, 8 cm distal to the duodenojejunal angle, was found in one patient. Colonic necrosis limited to the splenic flexure or to the left transverse colon was seen in two patients, in one with perforation. Two further patients also had splenic infarction and distal necrotizing pancreatitis, respectively.
Surgical Procedure
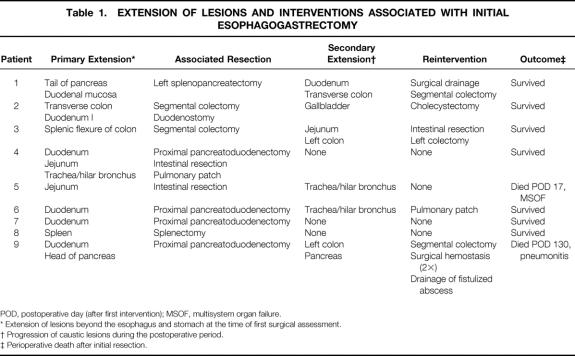
Total esophagogastrectomy without thoracotomy was performed by a stripping procedure through a combined abdominal and cervical approach in eight patients, as previously described (Table 1) . 7 Esophagectomy had to be performed through a right thoracotomy in one patient with tracheobronchial necrosis, which was repaired with a pulmonary patch. 8 Four patients underwent proximal pancreatoduodenectomy for duodenal or pancreatic necrosis, associated with the resection of 40 cm of jejunum in one patient. The main pancreatic duct was sealed by the injection of a polymer (Ethibloc, Ethnor Laboratories, Paris, France), and the pancreatic remnant was closed with an autostapling device. All four patients underwent hepaticojejunostomy. Duodenostomy was performed in one patient with necrosis limited to the first duodenum. Resection and anastomosis were performed in one patient with isolated jejunal necrosis. Two colonic lesions were treated by resection and double colostomy. Left splenopancreatectomy and splenectomy were performed in two further patients.
Table 1. EXTENSION OF LESIONS AND INTERVENTIONS ASSOCIATED WITH INITIAL ESOPHAGOGASTRECTOMY
POD, postoperative day (after first intervention); MSOF, multisystem organ failure.
* Extension of lesions beyond the esophagus and stomach at the time of first surgical assessment.
† Progression of caustic lesions during the postoperative period.
‡ Perioperative death after initial resection.
Complications and Death
Five patients required at least one reintervention in the postoperative period (see Table 1). One patient with initial associated pancreatoduodenectomy underwent segmental colectomy on postoperative day 21 for colonic perforation resulting from progression of caustic injury. A necrotic-hemorrhagic pancreatitis developed, which required three reoperations for hemorrhage and drainage of a fistulized pancreatic abscess. One patient with mucosal necrosis of the duodenum underwent surgical drainage of a duodenal fistula and total resection of the transverse colon, including the hepatic flexure, on postoperative day 12. In a third patient, initial splenic flexure colectomy was completed, on postoperative day 4, by left colectomy and extensive intestinal resection, preserving 120 cm of terminal ileum. One patient had biliary peritonitis related to gallbladder necrosis. Tracheobronchial necrosis developed in two patients on postoperative days 11 and 17. One was treated with a pulmonary patch; the second died of multisystem organ failure before reintervention. A pancreatic fistula developed after proximal pancreatoduodenectomy and was treated medically. Finally, five severe pulmonary infections occurred and were treated.
Mean intensive care unit stay was 58.8 days (range 16–152) after the first operation.
Two patients died after the initial operation, one on day 17 of multisystem organ failure, and the other on day 130 of pulmonary infection.
Esophageal Reconstruction
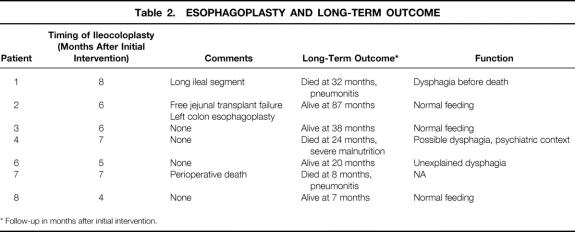
Mean follow-up of the seven survivors was 38 months (range 7–87). They all underwent retrosternal ileocolonic esophagoplasty 4 to 8 months after the initial operation (Table 2). The distal anastomosis of the plasty was tailored on the second duodenum four times and on the proximal jejunum three times because of previous pancreatoduodenectomy. In two patients who had had transverse colonic resection during the initial operation, the ileocolonic plasty was not long enough to reach the cervical esophagus. In the first, a free jejunal transplant was added to the plasty. Necrosis of this transplant led to the construction of a successful left colonic esophagoplasty. In the other, the plasty included a long ileal segment, of which the cervical end had to be revascularized from the subclavian vessels.
Table 2. ESOPHAGOPLASTY AND LONG-TERM OUTCOME
* Follow-up in months after initial intervention.
Late Death and Functional Results
Three patients died after reconstructive surgery, one in the postoperative period of pulmonary infection, and the other two 16 and 24 months after esophagoplasty of severe malnutrition and of pulmonary infection, respectively. Neither of these two patients had recovered normal feeding, in one in the context of severe psychiatric disorders. Among the four survivors, three eat normally. One patient has dysphagia, for which no cause has been found despite repeated radiologic and endoscopic examinations.
DISCUSSION
After severe caustic digestive injuries, patient survival is closely related to the delay between ingestion and surgical treatment. 9 Major metabolic disorders and extension of the caustic burns beyond the esophagus and the stomach largely account for the increased death rate found in patients who undergo delayed surgery. Upper digestive endoscopy is the most important examination and must be performed without delay. Tracheobronchial endoscopy must be performed before surgery whenever necrosis is seen on the upper two thirds of the esophagus to detect tracheobronchial necrosis, which would alter the surgical management. 8 Thoracomediastinoscopy is not as informative as endoscopic examination. Also, in the setting of chemical mediastinitis, it is contraindicated because it carries a prohibitive risk of tracheal or esophageal perforation and of pleural extension of the caustic injury. Unlike others, 10 we believe that ultrasound and abdominal CT scans are of little use either in establishing the indications for surgery in the early phase after ingestion or in defining the extent of lesions to adjacent organs. In addition, they are time-consuming and delay surgery in a situation where every hour counts. Moreover, the extent of abdominal lesions is much better assessed during surgery.
Neutralization studies by weak acid or alkali administration performed in canine models have shown reduced injury progression when administered early (within 5 to 30 minutes of caustic ingestion), as well as an absence of thermal effects. 11–13 However, such therapies are not yet used in the clinical setting. They do not appear to be applicable to patients who appear for treatment late and with already devastating injuries.
Extended esophagogastric necrosis, major metabolic disorders, or peritoneal signs warrant emergency resection of the necrotic esophagus and stomach to prevent extension of the injury to adjacent organs. 9 Comprehensive abdominal exploration is mandatory, and all injured organs must be resected during the first operation. Even minimal caustic lesions must be removed at that time because they invariably progress. A minimal resection followed by a planned second-look procedure is not recommended.
We are investigating the role of laparoscopy in the exploration and resective treatment of patients with caustic injuries. However, there are two caveats associated with this technique. First, it is not a substitute for a comprehensive abdominal exploration, particularly in the posterior aspects of the stomach and duodenum, where the most severe injuries are likely to be located. Second, it should not unduly extend the operative time in a situation where time is a major determinant of outcome.
The high surgical complication rate reported here and the need for reoperation were related to the ongoing progression of the caustic injury in all patients. There was no macroscopic indication to predict such outcomes at the time of the initial operation. Because the diagnosis of these complications is difficult in intensive care unit patients, repeat laparotomy must be readily performed when in doubt.
The only complication related to initial pancreatoduodenectomy was a pancreatic fistula, which was treated medically. The treatment of the pancreatic remnant in these patients is controversial. We prefer to obstruct the main pancreatic duct by a polymer and staple the pancreatic remnant. Selective intubation of the main pancreatic duct does not seem to provide good results. 5,6 Pancreaticojejunostomy is particularly hazardous in these patients because of the combined presence of soft healthy pancreatic tissue, peritoneal inflammation, and frequent hemodynamic instability in the postoperative period. However, successful performance of pancreaticojejunostomy in this situation has been reported. 3
Considering the severity of the lesions, the extent of initial resections, and the number of reoperations, the perioperative death rate was remarkably low. In one patient who died on day 17, resection could not control septic shock and the development of multisystem organ failure. The other death occurred in a patient who died of repeated pulmonary infections after several reinterventions for necrotic-hemorrhagic pancreatitis.
With regard to reasonable limits of resectability, we refrain from performing resection only if there is massive intestinal necrosis. In this situation, in the unlikely event of patient survival, the long-term outcome would be jeopardized by nutritional and reconstructive issues.
Esophageal reconstruction in these patients presents distinctive features. After esophagogastrectomy, we usually performed the distal anastomosis on the second duodenum. After pancreatoduodenectomy, it has to be performed on the jejunum, a routine procedure for others. 14 If extensive, colonic resection impairs the construction of the esophagoplasty. In this series, the lack of a sufficient length of colon as a result of previous colonic resections was handled in two ways: either a long segment of terminal ileum was used and revascularized at its cervical end, or the colonic transplant was extended by a free jejunal segment (albeit unsuccessfully).
In conclusion, while underlining our policy of early and aggressive surgical intervention for severe esophagogastric caustic burns, our results suggest that additional resection extended to adjacent abdominal organs, in particular the pancreas, does not carry a prohibitive risk of death. Massive intestinal necrotic injury represents the reasonable limit for resection. Progression and secondary extension of caustic burns is a common but unpredictable event; when in doubt, reexploration is indicated. With extensive colonic lesions, wide initial resections compromise the reconstruction and require vascular surgery for atypical transplants.
Footnotes
Correspondence: Pierre Cattan, MD, Service de Chirurgie Digestive, Hôpital Saint-Louis, 1 Avenue Claude Vellefaux, 75010 Paris, France.
Accepted for publication November 16, 1999.
References
- 1.Celerier M, Gayet B. Les brûlures par ingestion de caustique. In: Celerier M, Gayet B, eds. Les Traumatismes de l’Oesophage. Paris: Arnette-Blackwell; 1995: 9–64.
- 2.Ganepola GA, Bhuta K. A case of total esophago-gastro-duodeno-jejunectomy and partial pancreatectomy for lye burns, and reconstruction with colon interposition. J Trauma 1984; 24:913–916. [DOI] [PubMed] [Google Scholar]
- 3.Wu MH, Lai WW, Hwang TL, et al. Surgical results of corrosive injuries involving esophagus to jejunum. Hepato-Gastroenterology 1996; 43:846–850. [PubMed] [Google Scholar]
- 4.Wu MH, Lai WW. Surgical management of extensive corrosive injuries of the alimentary tract. Surg Gynecol Obstet 1993; 177:12–16. [PubMed] [Google Scholar]
- 5.Guth A, Pachter L, Albanese C. Combined duodenal and colonic necrosis: an unusual sequela of caustic ingestion. J Clin Gastroenterol 1994; 19:303–305. [DOI] [PubMed] [Google Scholar]
- 6.Losanoff J, Kjossef K. Multivisceral injury after liquid caustic ingestion. Surgery 1996; 119:720. [DOI] [PubMed] [Google Scholar]
- 7.Gossot D, Sarfati E, Celerier M. Early blunt esophagectomy in severe caustic burns of the upper digestive tract. J Thorac Cardiovasc Surg 1987; 94:188–191. [PubMed] [Google Scholar]
- 8.Sarfati E, Jacob L, Servant JM, et al. Tracheobronchial necrosis after caustic ingestion. J Thorac Cardiovasc Surg 1992; 103:412–413. [PubMed] [Google Scholar]
- 9.Sarfati E, Gossot D, Assens P, Celerier M. Management of caustic ingestion in adults. Br J Surg 1987; 74:146–148. [DOI] [PubMed] [Google Scholar]
- 10.Oakes DD. Reconsidering the diagnosis and treatment of patients following ingestion of liquid lye. J Clin Gastroenterol 1995; 21:85–86. [DOI] [PubMed] [Google Scholar]
- 11.Homan CS, Maitra SR, Lane BP, Thode Jr, Finkelshteyn J, Davidson L. Effective treatment for acute alkali injury to the esophagus using weak-acid neutralization therapy: an ex-vivo study. Acad Emerg Med 1995; 2:952–958. [DOI] [PubMed] [Google Scholar]
- 12.Homan CS, Singer AJ, Henry MC, Thode HC Jr. Thermal effects of neutralization therapy and water dilution for acute alkali exposure in canines. Acad Emerg Med 1997; 4:27–32. [DOI] [PubMed] [Google Scholar]
- 13.Homan CS, Singer AJ, Thomajan C, Henry MC, Thode HC Jr. Thermal characteristics of neutralization therapy and water dilution for strong acid ingestion: an in-vivo canine model. Acad Emerg Med 1998; 5:286–292. [DOI] [PubMed] [Google Scholar]
- 14.Cerfolio RJ, Allen MS, Deschamps C, et al. Esophageal replacement by colon interposition. Ann Thorac Surg 1995; 59:1382–1384. [DOI] [PubMed] [Google Scholar]