Abstract
Objective
To determine the clinical significance of a molecular assay based on the reverse transcriptase polymerase chain reaction (RT-PCR) for the presence of micrometastatic melanoma cells in sentinel lymph nodes (SLNs).
Summary Background Data
Routine histologic examination of lymph nodes often underestimates the presence of micrometastatic disease. The authors have previously shown that an RT-PCR assay designed to detect melanocyte-specific expression of the tyrosinase gene could be used to define a population of patients at higher risk for both recurrence and death compared with routine hematoxylin and eosin (H&E) histology. In this study, the authors used the tyrosinase RT-PCR assay in a patient population examined by a more detailed histologic analysis, including S-100 immunohistochemistry.
Methods
Patients underwent lymphatic mapping and SLN biopsy. SLN specimens were bivalved, and half of each specimen was serially sectioned and examined by routine H&E histology and S-100 immunohistochemistry. The other half of each specimen was analyzed by a nested RT-PCR assay.
Results
Hematoxylin and eosin histology detected metastatic disease in 36 (16%) of the 233 patients tested. S-100 immunohistochemistry detected micrometastatic disease in another 16 patients, and 114 (63%) of 181 patients with histology-negative nodes had positive findings on RT-PCR. There were significant differences between PCR-positive and PCR-negative patient groups in Breslow thickness, Clark level, and the presence of ulceration of the primary tumor, factors that have been shown to correlate with recurrence and survival.
Conclusions
These results suggest that RT-PCR can increase the sensitivity of detection of metastatic melanoma cells in SLNs over the current standard methods, including H&E histology and S-100 immunohistochemistry. Further long-term follow-up is needed to detect actual differences in recurrence and overall survival.
The most powerful predictor of survival for melanoma and other solid tumors is the status of regional lymph nodes. Once nodal disease develops, prognostic factors based on the primary tumor offer little help in predicting recurrence rates and overall survival. For patients with malignant melanoma, the 5-year survival rate decreases 40% with the development of stage III disease (nodal metastases). Therefore, emphasis should be placed on obtaining an accurate nodal staging to allow better patient management, particularly with the approval of interferon alfa-2b for adjuvant treatment in patients with stage III melanoma. 1
In the past, elective lymph node dissection was performed to obtain nodal staging information. All lymph nodes in the lymphatic drainage basin were removed for pathology examination for the presence of micrometastases. Elective lymph node dissection is a radical surgical procedure, and complications such as lymphedema and neuropathy may directly affect the patient’s quality of life after surgery. More importantly, approximately 80% of patients who undergo elective lymph node dissection have no evidence of metastases or subsequent recurrence; therefore, most patients do not directly benefit from this procedure.
Recently, the combination of several conservative diagnostic and surgical techniques to harvest and to analyze more closely sentinel lymph nodes (SLNs) has revolutionized the staging and treatment of patients with malignant melanoma. Originally proposed by Morton et al, 2,3 the SLN is defined as the first lymph node in the regional lymphatic basin that drains the primary tumor. Preoperative lymphoscintigraphy, intraoperative lymphatic mapping, and selective SLN biopsy can be sequentially performed to harvest one or two SLNs. Several reports have confirmed that the SLN is the first node that receives metastatic melanoma cells and that the SLN reflects the metastatic status of the entire lymphatic basin. 4–8 Thus, if the SLN lacks metastases, the whole basin will also be free of metastases. This strategy allows pathologists to focus on just 1 or 2 lymph nodes instead of 20 to 30 nodes from an elective lymph node dissection, making it possible to acquire accurate nodal staging information with a more detailed examination of the SLNs.
Standard procedures for the pathologic examination of regional lymph nodes involve serial sectioning at 1- to 4-mm intervals, staining of 3-μm sections with hematoxylin and eosin (H&E), and inspection for melanoma cells among background lymphocytes under microscopy. Pathologists determine the presence or absence of metastases, and clinicians make their treatment decisions based on the results of this superficial examination, which typically studies less than 1% of the submitted nodal tissue. However, studies show that metastatic melanoma can invade the lymph node with a very low volume and it can also localize to a small region in the node, not necessarily the central cross-section of the node. 9
Immunohistochemistry staining with antibody to the S-100 protein, a relatively specific marker for melanoma cells, can increase the sensitivity of detecting melanoma cells in lymph nodes tenfold over H&E staining alone, 10 to approximately 1 melanoma cell in a background of 105 normal lymphocytes. Since 1995, our program has incorporated the use of S-100 immunohistochemistry staining into routine melanoma SLN examinations. Our data show that the addition of S-100 immunohistochemistry staining increased the incidence of a positive finding for metastatic melanoma by 20%. 11 However, there are still some patients with negative S-100 immunohistochemistry and H&E-staining lymph nodes who have had recurrence and died of metastatic melanoma. Identifying this subgroup of patients who may have “submicroscopic” metastatic disease requires an even more sensitive method.
Tyrosinase is a key enzyme in the biosynthesis of melanin in normal melanocytes and melanoma cells. 12 It is not expressed in normal lymph nodes and peripheral blood. The presence of tyrosinase mRNA in lymph nodes or peripheral blood can be used to indicate the presence of metastatic cells. Smith et al 13 first designed a reverse transcription (RT) coupled to a nested polymerase chain reaction (PCR) assay to detect tyrosinase mRNA as a marker for circulating melanoma cells in peripheral blood. We expanded and modified this method for use in SLNs. 14 Previous data suggest that the RT-PCR assay can identify 1 melanoma cell in 106 to 107 background normal cells. 14,15
Our laboratory has recently shown that the nested RT-PCR assay for the detection of tyrosinase mRNA as a marker for the presence of metastatic melanoma cells in SLNs identified a subpopulation of patients whose nodes were negative by routine H&E histology but who overall had a significantly increased risk of recurrence and death from metastatic melanoma. 16 Further, we showed that in both univariate and multivariate regression analyses, the RT-PCR status of the SLN was a statistically significant predictor of disease-free survival. However, one problem with the previous studies was that they were performed at a time when S-100 immunohistochemistry was not routinely performed, and so there could have been patients with micrometastatic disease who might not have been missed by the current methods using S-100.
Does the increase in sensitivity for the PCR assay really have any clinical relevance for patients who are at risk for metastatic melanoma, especially when compared with the now-standard use of S-100 immunohistochemistry? The current study, which was performed on a separate population of patients in whom S-100 immunohistochemistry was a component of the routine histologic analysis of the SLN, was designed to investigate the utility of the RT-PCR assay for tyrosinase mRNA as a marker for the detection of metastatic melanoma cells in SLNs.
METHODS
Study Design
A prospective cohort study was conducted to observe patients with clinical stage I or II cutaneous malignant melanoma who were at risk of local, regional, or systemic metastasis. Patients underwent preoperative lymphoscintigraphy, intraoperative lymphatic mapping, and SLN biopsy, followed by wide local excision of the primary tumor. SLNs were bisected, and each half was submitted for histology (H&E and S-100 immunohistochemistry) or RT-PCR, randomly. Patients were followed up for melanoma recurrence and survival.
Patient Population
Consecutive patients with malignant melanoma were recruited into this study from December 1995 to December 1997 from the Cutaneous Oncology Program, H. Lee Moffitt Cancer Center and Research Institute at the University of South Florida in Tampa. Institutional review board approval was obtained before the first patient was enrolled. Each patient gave consent for the study and was willing to participate voluntarily. All patients met the following inclusion and exclusion criteria:
1. A biopsy-confirmed diagnosis of melanoma within 3 months
2. A primary tumor Breslow thickness greater than 0.76 mm, unless there were other high-risk factors for metastasis
3. No grossly palpable disease present on physical examination
4. No signs or symptoms of local, regional, or systemic metastatic disease
5. No evidence of multiple primary melanomas.
These criteria ensured that all patients enrolled in the study were at clinical stage I or II. Patients who had an unsuccessful SLN biopsy or PCR test (negative for the β-actin control) were removed from the study.
A final cohort of 233 patients who met all the criteria was followed for melanoma recurrence and survival.
Preoperative Lymphoscintigraphy
All patients underwent preoperative lymphoscintigraphy to determine the actual lymphatic basin(s) at risk for metastases and the approximate number and location of SLN(s), as described previously. 17 Briefly, filtered (0.2-micron filter) technetium-labeled sulfur colloid (Syncor, Inc., Tampa, FL) was injected around the primary tumor or the previous biopsy site. Dynamic and delayed images were obtained to show the lymphatic drainage pattern of the primary tumor, as well as the anatomic relations of potential SLNs. An intradermal tattoo was used to mark the location of potential SLNs.
Intraoperative Lymphatic Mapping and SLN Biopsy
Patients were taken to the operating room for intraoperative lymphatic mapping and SLN biopsy under general anesthesia. A combination lymphatic mapping technique was used that included a vital blue dye (Lymphazurin; USSC, Norwalk, CT) and the radiocolloid to identify the SLN during surgery. The SLN harvest was performed 2 to 24 hours after lymphoscintigraphy, so the radioactivity in SLNs was still detectable and reinjection of radiocolloid was avoided. Vital blue dye was injected around the primary tumor or previous biopsy site at the beginning of the surgical procedure. Ten minutes after the injection of blue dye, a small incision was made at the site of the intradermal tattoo. Lymph nodes with a blue-stained afferent lymphatic or containing blue dye were harvested as SLNs. “Hot” lymph nodes that had appropriate ratios of radioactivity versus background (>3:1 in vivo) or versus a neighboring non-SLN (>10:1 ex vivo), detected by a hand-held gamma probe, were also harvested as SLNs. Subsequently, the primary tumor was treated with a 1.0-cm wide local excision for melanomas less than 1.0 mm thick or a 2.0-cm wide local excision for all other tumor thicknesses.
Histologic Examination
The SLNs were bisected and analyzed by both routine histology and RT-PCR. Half of each SLN was submitted to the pathology department and processed for a routine pathology examination. The specimens were sectioned at 2- to 3-mm intervals and submitted for paraffin embedding. Each block was sectioned at one to three levels, depending on the size of the tissue in the block, and stained with H&E. If no melanoma cells were found with H&E staining, immunohistochemistry staining with S-100 antibody was performed using an avidin–biotin complex immunoperoxidase technique with diaminobenzidine chromogen. For specimens that were negative by H&E but positive by immunohistochemistry, the H&E slides were carefully reviewed again or even more blocks were inspected to verify the presence of metastatic melanoma cells. Only specimens that were confirmed positive by H&E staining were reported as histology-positive.
RT-PCR Examination
The other half of each SLN was processed for RNA extraction and RT-PCR, as previously described. 16 Briefly, samples were sent immediately from the operating room to the laboratory, where they were trimmed of any external fat and rapidly frozen at −80°C. Total RNA was extracted from the entire specimen by a phenol-guanidinium thiocyanate method. A cDNA library was constructed using oligo-dT as the primer for reverse transcription. A separate PCR assay for the mRNA of the β-actin housekeeping gene was performed to verify general mRNA integrity. A nested PCR for the detection of tyrosinase cDNA was conducted on β-actin–positive samples to determine lymph node metastatic status. The first round of PCR (30 cycles) generates a 284-base pair (bp) DNA fragment; the second round (30 cycles) with nested primers generates a 207-bp product. The second-round PCR products were analyzed by electrophoresis in a 10% TBE gel (BioRad, Hercules, CA) and stained with ethidium bromide. If an SLN sample produced the 207-bp fragment, it was considered PCR-positive.
Adjuvant Therapy
Patients who had histology-positive nodes by H&E or S-100 staining were offered completed lymph node dissection or interferon alfa-2 therapy or the opportunity to enter other clinical trials for stage III disease. PCR results were not used for clinical decision making, and all patients whose samples were negative by routine histology were observed.
Follow-Up Schedule
Patients were followed up on a regular schedule after surgery, at least every 3 to 6 months for the first and second years after surgery and yearly afterwards. Patients were checked carefully by physical examination, chest x-rays, or other diagnostic tools, if necessary. Recurrence was confirmed by examining charts or by contacting referring physicians.
Statistics
Survival functions were generated for relapse-free survival using the product-limited method of Kaplan-Meier. 18,19 Overall survival was calculated from the date of diagnosis to the date of death. Relapse-free survival was calculated from the date of diagnosis to the date of first recurrence. Patients not experiencing these events were considered censored at the date of last contact. The distribution of each variable was evaluated, and measures of central tendency and variance were estimated. Univariate and multivariate regression analyses were performed with prognostic variables based on the primary tumor, such as tumor thickness, Clark level, ulceration, and primary melanoma location, as well as clinical variables such as age, sex, histology, and RT-PCR status of the SLN. 20 Chi-square statistics were used for comparing differences among different categories, which were formed according to PCR and histology status. An α level of 0.05 and 95% confidence intervals (CIs) were used throughout the analysis.
RESULTS
Patient Demographics
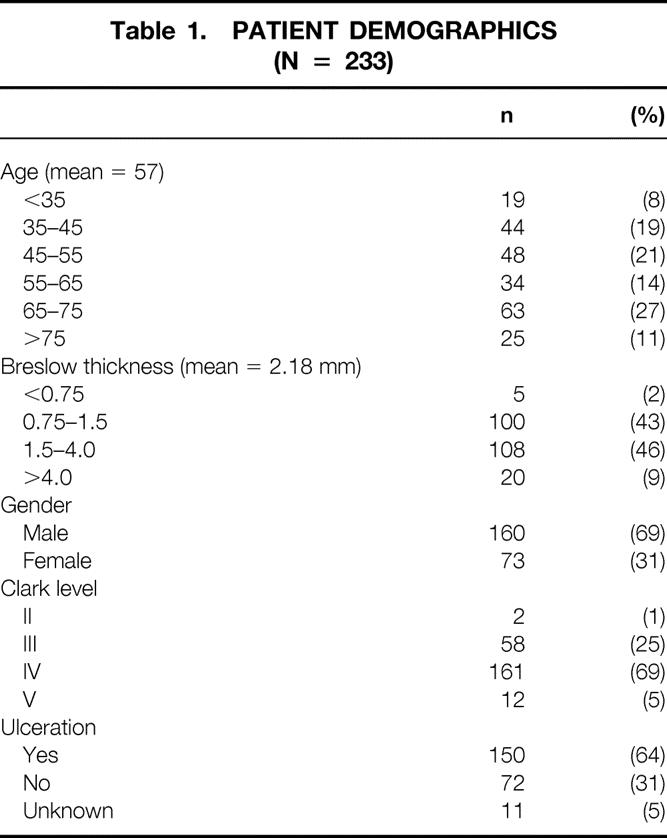
A summary of demographic data for the 233 study patients is shown in Table 1.
Table 1. PATIENT DEMOGRAPHICS (N = 233)
Pathology Examination and RT-PCR Examination
Two separate dermatopathologists (J.M., L.F.G.) read each H&E and immunohistochemistry slide. In the routine examination under H&E staining, 36 patients were found to have metastatic melanoma in one or more SLNs. All of these patients also had positive nodes by S-100 immunohistochemistry. In addition, another 16 patients had positive nodes on S-100 immunohistochemistry staining after metastatic cells had not been detected by the initial H&E staining. On reexamination and in some cases further sectioning, the presence of metastatic melanoma cells was verified in all of these patients. Therefore, the total rate of histology positivity was 22% (52/233) in our study population. S-100 immunohistochemistry staining was responsible for the identification of 31% (16/52) of the patients with metastatic disease. This disease would have been missed and the patients might have been staged inaccurately if elective lymph node dissection with a more superficial examination of all the nodes in the regional basins had been performed or if immunohistochemistry was not performed in the SLN.
Using RT-PCR for tyrosinase mRNA to detect metastatic melanoma cells in SLNs, 163 of 233 (70%) patients had positive nodes. Of 52 patients with histology-positive nodes, 49 (94%) also had PCR-positive nodes. Of the total of 163 patients with presumed evidence of metastatic disease in their SLNs, routine H&E pathology identified only 22% (36/163) of the patients with SLN metastases. Immunohistochemistry staining identified an additional 10% (16/163) of patients with metastatic disease, but the bulk of the patients with presumed nodal disease (68%) was detected only by the RT-PCR assay.
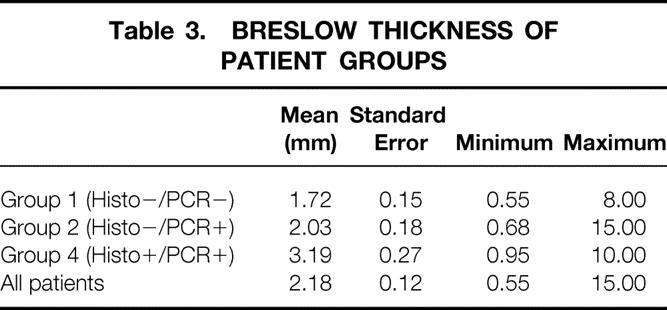
Patients were categorized by histology (both H&E and S-100 immunohistochemistry) and the RT-PCR status of their SLNs into four groups: group 1 (n = 67), negative on both histology and PCR; group 2 (n = 114), histology-negative but PCR-positive; group 3 (n = 3), histology-positive but PCR-negative; and group 4 (n = 49), positive on both histology and PCR for metastatic melanoma.
An independent t test, performed to compare differences in Breslow thickness, found a significant difference (P < .01) between patients with PCR-positive and PCR-negative nodes (Tables 2 and 3). The difference in Breslow thickness between PCR-positive and PCR-negative nodes was 0.68 mm (95% CI, 0.15–1.21 mm).
Table 2. CORRELATION OF RT-PCR POSITIVITY WITH BRESLOW THICKNESS
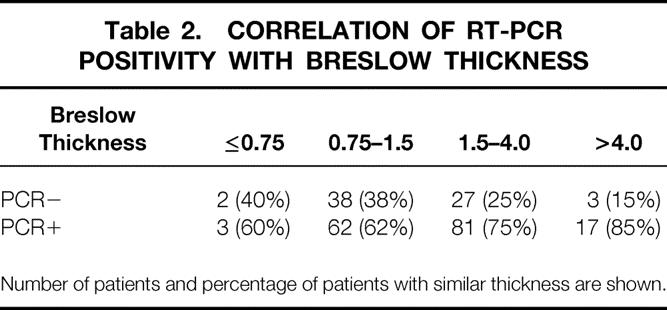
Number of patients and percentage of patients with similar thickness are shown.
Table 3. BRESLOW THICKNESS OF PATIENT GROUPS
Logistic regression analysis found that PCR positivity was correlated with tumor thickness, Clark level, and tumor ulceration (Tables 2–8 As the thickness of the primary tumor increased by 1 mm, the probability of getting a positive RT-PCR result increased by a factor of approximately 1.04.
Table 4. CORRELATION OF RT-PCR POSITIVITY WITH CLARK LEVEL
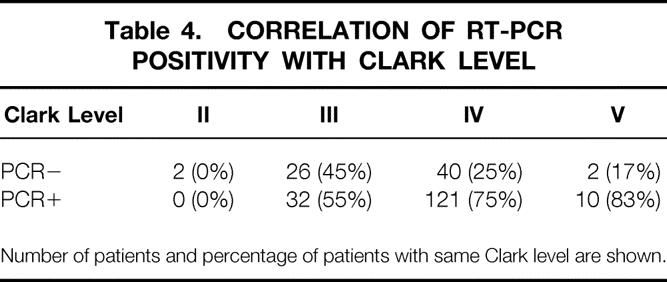
Number of patients and percentage of patients with same Clark level are shown.
Table 5. CORRELATION OF RT-PCR POSITIVITY WITH TUMOR ULCERATION
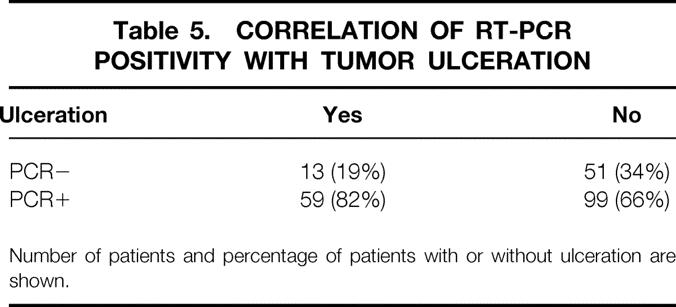
Number of patients and percentage of patients with or without ulceration are shown.
Table 6. CLARK LEVEL OF PATIENT GROUPS
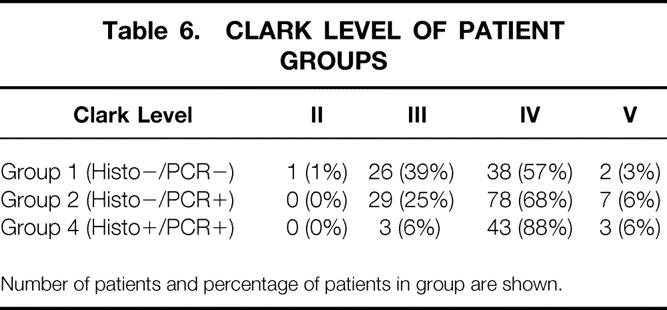
Number of patients and percentage of patients in group are shown.
Table 7. TUMOR ULCERATION OF PATIENT GROUPS
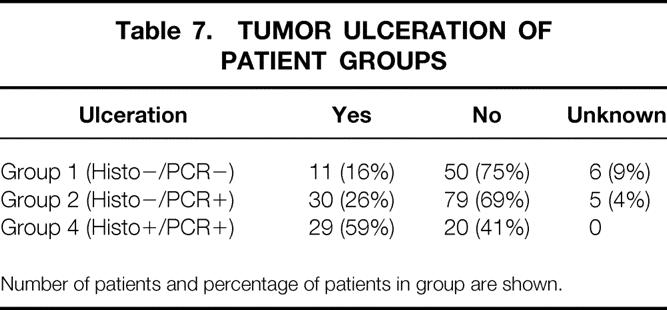
Number of patients and percentage of patients in group are shown.
Table 8. LOGISTIC REGRESSION OF PCR POSITIVITY FOR PRIMARY TUMOR VARIABLES
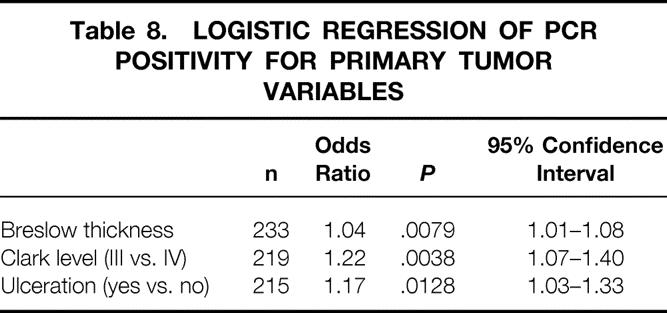
Follow-Up
A total of 224 patients had at least 3 months of follow-up since their SLN biopsy, and the mean follow-up time for the whole study population was 20 months. Thirty patients had documented recurrence after their SLN biopsy (3–38 months after surgery) and 14 patients had died of melanoma during this follow-up interval. A breakdown of patients with respect to their categories is given in Table 9. One patient (1.6%) in group 1 had recurrence and died of melanoma. In group 2, 11 (10.2%) patients had recurrence, and 4 (3.7%) of them died of disease. Eighteen (36%) patients in group 4 had recurrence, and 9 (18%) of these patients died of metastatic disease. Of the 12 patients with negative nodes by histology in whom recurrence developed later, 11 (92%) had nodes that were positive by RT-PCR.
Table 9. RECURRENCE AND SURVIVAL FOR PATIENT GROUPS
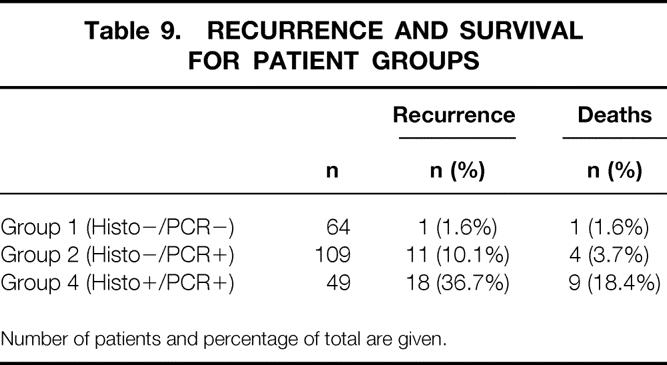
Number of patients and percentage of total are given.
A Kaplan-Meier survival analysis conducted for disease-free survival found P values of .06 and .00005 between groups 1 and 2 and between groups 2 and 4, respectively.
DISCUSSION
Selective SLN biopsy enables pathologists and investigators to focus their search for metastatic cells on a small number of lymph nodes that are most likely to harbor metastatic disease; this results in the acquisition of more accurate staging information. The correlation between SLN histology status and clinical outcome has been well established. However, a significant percentage of patients have recurrence after their SLNs are found to be negative with routine histologic examination. In this study, we used RT-PCR as a more sensitive method to detect “submicroscopic” metastatic melanoma cells.
From the clinician’s viewpoint, a patient with a thicker primary tumor, higher Clark level, and ulceration is more likely to have metastatic lymph nodes. 21 Our data show that patients who are clinically at high risk for metastases are more likely to have PCR-positive nodes as well. For each 1-mm increase in Breslow thickness of the primary tumor, the probability of finding a PCR-positive SLN increased by a factor of 1.04 (95% CI, 1.01–1.08). Patients with a Clark level IV tumor were 1.17 (95% CI 1.03–1.33) times more likely to have a PCR-positive SLN than patients with a Clark level III tumor. Patients with an ulcerated primary tumor were 1.22 (95% CI, 1.07–1.40) times more likely to have a PCR-positive SLN.
Routine histologic examination of SLNs examines only a very small amount of tissue, and this limited examination may miss low-volume micrometastatic disease. A small retrospective study conducted at our center and at the M.D. Anderson Cancer Center reexamined SLN samples from patients who had histology-negative nodes (H&E) but nodal recurrences. 22 Serial sectioning and immunohistochemistry staining demonstrated that 10 of 15 (66%) patients had metastatic melanoma cells in the SLN that were missed by the original routine histologic examination. However, even with the use of more extensive routine methods, the presence of metastatic cells could not be demonstrated in five patients (33%) in whom recurrences developed. In our present data, of the 12 patients whose nodes were negative by histology (including serial sectioning and immunohistochemistry) and in whom recurrence developed, 11 (92%) had positive nodes by RT-PCR. This finding implies that the RT-PCR assay detects clinically relevant disease, because these patients suffered recurrent disease during their follow-up.
In addition to one patient who had recurrence after a PCR-negative SLN, three patients in the current study had histology-positive but PCR-negative nodes. A possible explanation for these apparent PCR false-negative results is that tyrosinase might not be expressed by a few melanoma tumors. Another possibility is that localized metastatic tumor cells were present only in the half submitted to the pathology department, and thus a sampling error occurred. However, all of these nodes were grossly negative, and any sampling errors would be expected to occur randomly in both directions.
Our data support the use of a highly sensitive RT-PCR assay as a complement to routine histologic methods for detecting melanoma micrometastases. In a previous report, 16 we showed that the tyrosinase RT-PCR results correlated with disease-free and overall survival, although in that study of a separate cohort of 114 patients, immunohistochemistry was not performed as part of the routine histology protocol. With a mean follow-up of 24 months, there was a significant difference in both disease-free and overall survival between patients whose nodes were histologically negative but PCR-positive and those whose nodes were negative both histologically and on PCR.
In the current study of 233 patients, the median follow-up is only 20 months, and the survival differences between patients with nodes negative on histology and either positive or negative on PCR has not quite reached significance (P = .06). However, the incorporation of S-100 immunohistochemistry into the routine histology protocol has detected disease in some patients that would have been missed by H&E staining alone, thus decreasing the number of patients at risk for recurrence in the histology-negative groups. Longer follow-up might be needed to demonstrate statistically significant differences in this cohort.
A major question that remains is whether patients with PCR-positive nodes only will benefit from more aggressive treatment. With short follow-up, most of the patients with PCR-positive nodes only with this one marker have not had recurrence or died of metastatic melanoma, suggesting that there may be a large percentage of “biologic false-positives” and thus PCR might be too sensitive for clinical use. The overall relapse rate for patients with PCR-positive nodes was approximately 18%; that for patients with histology-positive nodes was approximately 36%. However, another explanation may be that the metastatic load is in such low volume that the disease is confined to the SLN, and performing the SLN harvest renders the patient free of disease. With this hypothesis, the SLN biopsy might be a therapeutic procedure for patients with a low volume of nodal disease, compared with patients whose metastases have had more time to spread to other nodes in the basin or other systemic sites. In addition, the body’s immune system may be better able to take care of lower volumes of metastatic disease. Selective SLN biopsy coupled with RT-PCR may detect metastatic melanoma at an earlier stage, when further therapy is not necessarily beneficial.
An ongoing national multicenter randomized clinical trial, called the Sunbelt Melanoma Trial, is trying to determine whether SLN biopsy coupled with RT-PCR will identify patients who will benefit from therapies currently reserved for patients with nodal disease detected by standard methods. In this trial, patients with melanomas greater than 1.0 mm thick will undergo wide local excision, lymphatic mapping, and SLN biopsy. Each SLN will be examined with routine H&E, S-100 immunohistochemistry, and RT-PCR. If the SLN is negative by histology but positive by RT-PCR, the patients will be randomized into three different arms (observation, complete lymph node dissection, or complete lymph node dissection plus interferon alpha-2b). If it can be shown that patients with nodal disease detected by PCR only can benefit from more aggressive therapies, then PCR will undoubtedly be incorporated into the standard of care for patients with melanoma in the near future.
Discussion
Dr. Kelly McMasters (Louisville, Kentucky): Dr. Reintgen and his colleagues are to be congratulated for pioneering the use of PCR analysis for the staging of melanoma. The present study confirms and extends their prior experience and helps usher in a new era in which molecular staging of cancer is used to help make treatment decisions.
The results of the Sunbelt Melanoma Trial confirm the experience of Dr. Reintgen and his colleagues with PCR testing of sentinel nodes. The Sunbelt Trial presently has over 1200 patients from more than 60 participating centers. In this study, however, we are using tyrosinase plus three additional markers, MART-1, MAGE-3, and gp100, to investigate molecular staging of sentinel nodes. A positive PCR test in the Sunbelt Trial is defined as the presence of tyrosinase plus at least one other marker positive for confirmation.
I’m going to show an update of the data that Dr. Reintgen showed from the Sunbelt Trial. This is with now only 9 months of median follow-up from the study, but you can see that the curves are starting to separate even further with a significant difference between the patients whose only evidence of disease is the presence of PCR positivity in their sentinel node. You can also see that the patients who are histologically and PCR-negative have an extremely good prognosis.
So one important goal of molecular staging is to identify subgroups of patients with occult metastatic disease who may benefit from additional treatment. But I think an even more important goal is to identify a subgroup of patients who have such a good prognosis that they don’t need any additional adjuvant therapy. That is, those patients who are histologically and PCR-negative. In Dr. Reintgen’s study, a total of 70% of the patients were found to be positive by either histology or immunohistochemistry or PCR testing in the sentinel node. Over 60% of the patients with primary tumors less than 1.5 mm thick were positive by PCR testing. It’s clear that the PCR testing overestimated, to some degree, the number of patients who would be expected to recur. So I have several questions:
While the sensitivity of PCR testing for tyrosinase is quite good, it seems that the specificity may be lower. How often is tyrosinase positive in the lymph nodes from nonmelanoma patients, that is, the negative control patients that you have tested?
Have you begun to use other markers in addition to tyrosinase in order to improve the specificity of the assay? As you know, in the Sunbelt Trial with the additional markers, the total rate of sentinel node positivity is 55%, that is, 25% that are histologically or immunohistochemically positive and about 30% that are positive by PCR only.
The next question: in your study, sentinel nodes that were positive by immunohistochemistry had to be confirmed by routine H&E staining in order to be called truly positive for cancer. As you know, we often see, in some slides with S100 staining, a few cells that light up with positive staining, and it has become a significant issue for pathologists and surgeons around the country. How do you deal with the issue when you see just a couple of cells that light up as positive on immunohistochemistry?
And, finally, because of your work, many patients and surgeons are starting to ask whether they can order sentinel lymph node PCR testing outside of a clinical trial. What’s your advice to them?
I thoroughly enjoyed the manuscript. Dr. Reintgen and his colleagues continue to lead the way in developing innovative approaches to the treatment of melanoma and this paper represents another in a long list of significant contributions.
Dr. Douglas R. Murray (Atlanta, Georgia): I wish to thank Dr. Reintgen for inviting me to discuss this fine paper and for giving me an opportunity to review the manuscript, which does embody several fine points on technique and will be appreciated by all. We deeply appreciate Doug’s untiring efforts to explore innovative procedures and set clinical standards in the management of melanoma.
This study employs a very sensitive amplified reverse transcriptase-nested polymerase chain reaction method to detect, if you will, submicroscopic disease.
Of 233 cases of primary melanoma examined, 52 cases, or 22%, were lymph node-positive by conventional means, immunohistochemistry and H&E staining; 31% of these were detected by immunohistochemistry, or 16 of the 52 cases, which, parenthetically, would have been missed by elective lymph node dissection procedures. One hundred and fourteen were positive by PCR alone (49%); therefore, 166 out of 233, or 71%, were positive. Of interest, only one of the 67 cases that were histologic-negative and PCR-negative recurred. Only 11 of 114 that were histologic-negative and PCR-positive recurred, or 10%.
It is of interest that 11 of these 12 patients that did recur were positive by PCR, even though negative by histology, suggesting that PCR does detect clinically relevant disease. Perhaps, as anticipated, 18, or 36% of the 49 patients that were histologic positive and PCR positive recurred. Nine, or 18%, of the 49 died. Nevertheless, the vast majority of the PCR-positive patients have not relapsed, suggesting a large percentage of biologic false-positives.
Therefore, the authors raise the question themselves: is PCR too sensitive for clinical use? There are disturbing reports from Europe, namely, Lukowsky at Humboldt University of Berlin (J Invest Dermatol 1999;113(4):554–9), who recently reported in a limited study of 24 patients that RT-PCR positivity was present in nonsentinel lymph nodes, an experience that challenges the concept of the sentinel lymph method. In seven of the 24 cases after a completion lymphadenectomy, positive RT-PCR was present in nonsentinel lymph nodes when conventional sentinel lymph node was negative. Five of these seven nonsentinel lymph nodes had gp100 and mRNA present. Their data suggests that the concept of sentinel node may miss micrometastases. Whether such micrometastases cause a recurrence remains to be clarified. Therefore, I have a few questions to pose:
Blue nodes and nodes with blue afferent lymphatics, and hot nodes meeting the proper background ratio criteria are considered sentinel lymph nodes. The original definition was the first lymph node in the regional lymphatic basin that drains the primary tumor. Do we need new terms for sentinel lymph nodes?
The false-positive rate appears high. What precautions might be employed to guard against high false-positive rates, such as a look at additional markers?
In the 114 cases that were PCR-positive alone, is it feasible—likely not—to study that half of the lymph node any further by histology? Did you look at gp100 or other markers?
Have you had an opportunity to study nonsentinel lymph nodes with this technique, given that completion lymphadenectomy was not carried out in this particular study?
Are we accumulating evidence or convincing data that the early identification of sentinel lymph node micrometastases improves the long-term outcome over simply a wait-and-watch practice with delayed lymph node dissection?
I congratulate Dr. Reintgen and his H. Lee Moffitt Cancer Center group in helping to lead us into the new molecular millennium.
Dr. William C. Wood (Atlanta, Georgia): I congratulate Doug and his colleagues again on conducting this trial and pushing the whole issue of what PCR really means when we find evidence of molecular components, at least, of these tumor cells.
The question comes, does it represent a really tiny burden of metastasis in these lymph nodes, or may it represent simply transitory cells passing through the lymph nodes? That, too, could simply represent burden of disease. It could be a surrogate. The person with more cells in transit, presumably, has a higher cell burden or more diseased cells; therefore, the possibility of detecting not even the cells, even breakdown products of the RNA DNA within the lymph nodes.
The question would then come: Have you simply found a molecular surrogate for tumor burden? And you mentioned in your presentation that this did correlate with level.
Have you done multivariate analysis to show that, yes, this breaks out well in univariate analysis, but in multivariate analysis, when you look at other standard measures of tumor burden, does it still hold?
Dr. Frederick L. Greene (Charlotte, North Carolina): The American Joint Committee on Cancer, on which I serve as executive chair, is very interested in this topic. As we develop our 6th edition of the staging manual, sentinel node technology will be considered.
My concern is, as some of the other discussants have mentioned, the oversensitivity of this test. Dr. Reintgen, since you served on the Task Force in melanoma, you are aware of this concern shared by the pathologists who serve on our Task Forces. I wish you would address that concern.
My final point is that 2 years ago at this meeting, we presented our work with PET scanning and melanoma. I postulated at that time that the PET scan may be a good way of showing nodal disease as well as metastases. I wonder if you would comment on that, and the use of PET in the staging of this disease.
Dr. Douglas S. Reintgen (Closing Discussion): Dr. Greene asked about staging, and certainly, I think that lymphatic mapping and sentinel biopsy and immunohistochemical staining will actually cause a reappraisal of the staging system for melanoma and for breast cancer. Dr. Balch is heading the melanoma work, and he has a paper in press in the journal Cancer with a proposed new staging system. So micrometastatic disease, identified with H&E and S-100 staining, will be part of that new staging system because it’s universally accepted that that is probably clinically relevant disease. I think it’s too early to put the RT-PCR data into the new proposed staging system.
He also had a question about PET scanning. With lymphatic mapping, sentinel node biopsy, and the PCR assay, we have the ability to identify one abnormal melanoma cell in a background of a million lymphocytes. I don’t think there is going to be a scan developed, whether it’s a PET scan, CT scan, or monoclonal antibody scan, that has that sort of sensitivity. So I think these molecular biology assays are here to stay.
Dr. Wood asked about burden of disease. Certainly he is right that this is detecting very low-volume disease, but it’s probably clinically relevant disease. We have done both a univariate and multivariate regression analysis in the initial report that was published in JAMA and have showed that, when examined with prognostic factors of the primary tumor as well as some clinical factors, that the PCR status of the sentinel node was an independent predictor of disease-free survival. We did not have enough deaths in the series to do the regression analysis for survival.
Dr. Murray suggested that maybe we are missing disease with the sentinel node biopsy. I think you would do that if you are doing the procedure wrong intraoperatively. But I believe the data suggests, for both melanoma and breast cancer, that with this technology, we are actually finding more disease than we would not find with just your routine elective lymph node dissection and a relatively superficial examination of the regional basin with one section of each node and just H&E stains. So the only way, I think, to miss disease is if you are doing the technique wrong. And you are finding much more disease with immunohistochemical staining than we would ordinarily with elective node dissection.
The last question concerns whether this technique is too sensitive?—that is, is it identifying too little disease? For the Sunbelt Melanoma Trial, the National Trial, we are taking a more conservative approach. The problem is that there is this phenomenon of intradermal nevus cell nests that we can find in normal lymph nodes, and certainly that would give a false-positive RT-PCR assay. We find that in about 5% of our cases. Some of the false-positive RT-PCR results are probably due to these benign nevus cell nests that you can find in the sentinel node in about 5% of cases.
That’s the reason why we really can’t take the whole node for PCR assay—we bivalve a node, give half to pathology and half to the PCR assay, and if we find nevus cells there, we know that we have a possible source of false positivity. We have taken a much more conservative approach with the National Trial, in that we are looking at a panel of four markers and nevus cells are negative with two of the other three markers. To be called positive on the trial, you have to be positive with the tyrosinase as well as positive with one of the other markers.
Footnotes
Correspondence: Douglas S. Reintgen, MD, Cutaneous Oncology Program, H. Lee Moffitt Cancer Center and Research Institute, University of South Florida, 12902 Magnolia Dr., Tampa, FL 33612.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
E-mail: reintgds@moffitt.usf.edu
Accepted for publication December 1999.
References
- 1.Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996; 14:7–17. [DOI] [PubMed] [Google Scholar]
- 2.Wong JH, Cagle LA, Morton DL. Lymphatic drainage of skin to a sentinel node in a feline model. Ann Surg 1991; 214:637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 1992; 127:392–399. [DOI] [PubMed] [Google Scholar]
- 4.Balch C, Soong SJ, Bartolucci AA, et al. Efficacy of an elective regional lymph node dissection of 1–4-mm-thick melanomas for patients 60 years of age and younger. Ann Surg 1996; 224:255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross MI, Reintgen DS, Balch C. Selective lymphadenectomy: emerging role for lymphatic mapping and sentinel node biopsy in the management of early melanoma. Semin Surg Oncol 1993; 9:219–223. [PubMed] [Google Scholar]
- 6.Uren RF, Howman-Giles RB, Shaw HM, et al. Lymphoscintigraphy in high-risk melanoma of the trunk: predicting draining lymph node groups, defining lymphatic channels and locating the sentinel node. J Nucl Med 1993; 34:1435–1440. [PubMed] [Google Scholar]
- 7.Reintgen DS, Cruse CW, Wells K, et al. The orderly progression of melanoma nodal metastases. Ann Surg 1994; 220:759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krag DN, Meijer SJ, Weaver DL, et al. Minimal-access surgery for staging of malignant melanoma. Arch Surg 1995; 130:654–658. [DOI] [PubMed] [Google Scholar]
- 9.Cochran AJ, Wen DR, Morton DL. Occult tumor cells in lymph nodes of patients with pathological stage I melanoma. Am J Surg Pathol 1988; 12:612–618. [DOI] [PubMed] [Google Scholar]
- 10.Cho KH, Hashimoto K, Taniguchi Y, et al. Immunohistochemical study of melanocytic nevus and malignant melanoma with monoclonal antibodies against S-100 subunits. Cancer 1990; 66:765–771. [DOI] [PubMed] [Google Scholar]
- 11.Messina J, Glass F. Pathologic examination of the sentinel lymph node. J Fla Med Assoc 1997; 84:153–156. [PubMed] [Google Scholar]
- 12.Kwon BS, Haq AK, Pomerantz SH, Halaban R. Isolation and sequence of a cDNA clone for human tyrosinase that maps at the mouse c-albino locus. Proc Natl Acad Sci USA 1987; 84:7473–7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith B, Selby P, Southgate J, et al. Detection of melanoma cells in peripheral blood by means of reverse-transcriptase and polymerase chain reaction. Lancet 1991; 338:1227–1229. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Heller R, VanVoorhis N, et al. Detection of submicroscopic lymph node metastases with polymerase chain reaction in patients with malignant melanoma. Ann Surg 1994; 220:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields KK, Elfenbein GJ, Trudeau WL, et al. Clinical significance of bone marrow metastases detected using the polymerase chain reaction in patients with breast cancer undergoing high-dose chemotherapy and autologous bone marrow transplantation. J Clin Oncol 1996; 6:1868–1876. [DOI] [PubMed] [Google Scholar]
- 16.Shivers S, Wang X, Li W, et al. Molecular staging of malignant melanoma: correlation with clinical outcome. JAMA 1998; 280:1410–1415. [DOI] [PubMed] [Google Scholar]
- 17.Berman C, Norman J, Cruse CW, et al. Lymphoscintigraphy in malignant melanoma. Ann Plast Surg 1992; 28:29–32. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53:457–481. [Google Scholar]
- 19.Mantel N. Evaluation of survival data and two new rank-order statistics arising in its consideration. Cancer Chemother Rep 1966; 50:163–170. [PubMed] [Google Scholar]
- 20.Cox D. Regression models and life tables. J R Stat Soc 1972; 34:187. [Google Scholar]
- 21.Balch CM, Cascinelli N, Drewiecki KT, et al. A comparison of prognostic factors world-wide. In: Balch CM, Houghton AN, Milton GW, Sober A, Soong S-J, eds. Cutaneous Melanoma, 2nd ed. Philadelphia: JB Lippincott; 1992: 188.
- 22.Gershenwald J, Colome MI, Mansfield P, et al. Patterns of recurrence following a negative sentinel lymph node biopsy in 243 patients with stage I or II melanoma. J Clin Oncol 1998; 16:2253–2260. [DOI] [PubMed] [Google Scholar]


