Abstract
Objective
To compare the experience and outcome in the management of hilar cholangiocarcinoma at one American and one Japanese medical center.
Summary Background Data
Controversies surround the issues of extent of resection for hilar cholangiocarcinoma and whether the histopathology of such cancers are similar between patients treated in America and in Japan.
Methods
Records were reviewed of 100 patients treated between 1980 and 1995 at the Lahey Clinic in the United States, and of 155 patients treated between 1977 and 1995 at Nagoya University Hospital in Japan. Selected pathologic slides of resected cancers were exchanged between the two institutions and reviewed for diagnostic concordance.
Results
In the Lahey cohort, there were 25 resections, 53 cases of surgical exploration with biliary bypass or intubation, and 22 cases of percutaneous transhepatic biliary drainage or endoscopic biliary drainage without surgery. In the Nagoya cohort, the respective figures were 122, 10, and 23. The overall 5-year survival rate of all patients treated (surgical and nonsurgical) during the study periods was 7% in the Lahey cohort and 16% in the Nagoya cohort. The overall 10-year survival rates were 0% and 12%, respectively. In patients who underwent resection with negative margins, the 5- and 10-year survival rates were 43% and 0% for the Lahey cohort and 25% and 18% for the Nagoya cohort. The surgical death rate for patients undergoing resection was 4% for Lahey patients and 8% for Nagoya patients. Of the patients who underwent resection, en bloc caudate lobectomy was performed in 8% of the Lahey patients and 89% of the Nagoya patients. Histopathologic examination of resected cancers showed that the Nagoya patients had a higher stage of disease than the Lahey patients.
Conclusions
In both Lahey and Nagoya patients, survival was most favorable when resection of hilar cholangiocarcinoma was accomplished with margin-negative resections. Combined bile duct and liver resection with caudate lobectomy contributed to a higher margin-negative resection rate in the Nagoya cohort.
Hilar cholangiocarcinoma has remained a challenge to surgeons because of its propensity for local invasion and its proximity to the portal vein, hepatic arteries, and liver parenchyma. Locally advanced disease at diagnosis and surgical inaccessibility have resulted in low resectability and poor survival. Recent advances in interventional radiology and endoscopy have made possible nonsurgical palliation of this disease. Consequently, some clinicians recommend that palliative biliary stenting supplant surgical resection as the standard treatment for patients with hilar cholangiocarcinoma. Although some patients with advanced disease or prohibitive surgical risk factors are best served by nonoperative biliary stenting, we believe most should be evaluated for surgical resection.
Recently, many institutions with expertise in the management of patients with hilar cholangiocarcinoma have reported higher resectability rates without increased surgical death rates. 1–12 Japanese surgeons 13–16 have reported resectability rates as high as 80% in patients treated by combined bile duct and liver resection with caudate lobectomy. Although surgical resection has gained acceptance during the past decade as the mainstay of therapy for hilar cholangiocarcinoma, the acceptance of combined liver resection with caudate lobectomy has been slow, especially by American surgeons. Moreover, the higher resectability and survival rates in Japanese reports have stirred debates as to whether the tumors in Japanese patients are of a more favorable histopathologic type or are detected at an earlier stage. The surgeons at Lahey Clinic and Nagoya University have a long shared an interest in the management of biliary malignancies and have gained from each other’s experience through the exchange of visiting surgeons. This study was prompted by one of these exchange visits. The aim of this study is to identify factors in our management approaches to hilar cholangiocarcinoma that might explain the reported differences in resectability and overall survival between American and Japanese patients.
METHODS
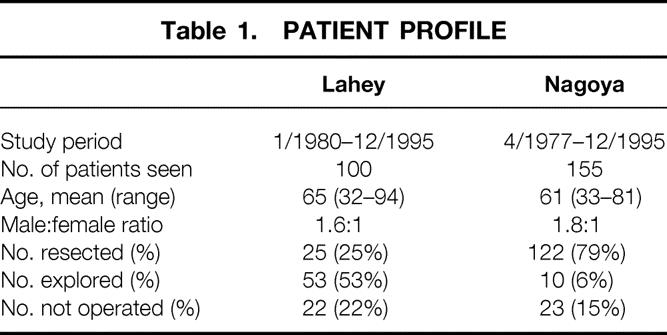
The Lahey cohort consisted of 100 patients seen and treated for hilar cholangiocarcinoma at Lahey Clinic between January 1980 and December 1995. There were 62 men and 38 women. The Nagoya cohort consisted of 155 patients referred for treatment of hilar cholangiocarcinoma at Nagoya University Hospital between April 1977 and December 1995. There were 100 men and 55 women (Table 1).
Table 1. PATIENT PROFILE
Patient data were obtained by retrospective chart review at the Lahey Clinic and by retrospective review of a prospectively gathered patient database at Nagoya University Hospital. Only patients with hilar cholangiocarcinoma were included in this study; patients with midduct, distal duct, or peripheral (intrahepatic) cholangiocarcinoma were excluded. Patient records were abstracted for data on demographics, preoperative interventions and staging investigations, types of therapeutic intervention, and treatment outcome. Follow-up data were obtained by direct patient or family contact, contact with referring physicians, or the tumor registry. Follow-up data were complete in all patients in both cohorts either to death or to a median of 5 years in living patients.
Statistical Analysis
Cohort data were analyzed using the Fisher exact test or chi-square analysis as appropriate. Probability was two-tailed, with P < .05 regarded as statistically significant. Survival curves were calculated using the Kaplan-Meier product-limit method. Statistical tests of significance across groups in each cohort were performed using the Mantel-Cox test at Lahey and the log-rank test at Nagoya, with P < 0.05 considered significant.
Pathologic Concordance
To establish concordance of pathologic diagnostic criteria and terminology between our two institutions, the glass histologic slides of resected tumors from selected patients were exchanged and reviewed by one surgical pathologist at each institution (J.M.D. at Lahey and K.O. at Nagoya). The exchanged slides (13 from Lahey and 20 from Nagoya) were chosen mainly from patients with superficial spreading carcinoma because there was concern over differences in interpretation of carcinoma in situ. After confirming pathologic diagnostic concordance, histology slides of all resected tumors were rereviewed within each institution by the same pathologists.
A negative resection margin was defined as the absence of any macroscopic or microscopic evidence of cancer, including carcinoma in situ and severe atypia, at the surgical resection margin.
Resectability Criteria
The general guidelines for resectability of hilar cholangiocarcinoma at the Lahey Clinic, between 1980 and 1995, were absence of lymph node, peritoneal, and discontiguous liver metastases, absence of vascular (portal vein or hepatic artery) invasion, and absence of extrahepatic adjacent organ invasion. The criteria for resectability at Nagoya University Hospital were more liberal. Resection was not precluded by the presence of locally advanced carcinoma with vascular invasion (main or unilateral portal vein and unilateral hepatic artery), regional lymph node metastases, or even paraaortic lymph node metastases. Adjacent extrahepatic organ invasion, likewise, was not an absolute contraindication to resection. The only absolute contraindication for resection at Nagoya University was bilateral hepatic arterial or bilateral portal venous encasement by cancer detected on preoperative angiography. Deviations from these resectability guidelines were observed at both institutions in cases of palliative resection.
RESULTS
Therapeutic Intervention
In the Lahey cohort, the overall resectability rate was 25%; 25 of 100 patients underwent surgical resection (Table 1). The mean age of the resection group was 62 years. Three patients in this group had limited metastatic disease found during surgery. Palliative resections were performed in these three patients because they were young and had good performance status. Fifty-three patients (53%) underwent surgical exploration, but their cancers were found to be unresectable at surgery. All patients in this group had locally advanced disease, and two patients had tissue-documented metastatic disease. Transtumoral T-tube intubation or hepaticojejunostomy for biliary decompression was performed in all patients in this group. The mean age for this group who underwent surgical exploration but had unresectable disease was 64 years. Twenty-two patients (22%) underwent percutaneous or endoscopic biliary stent placement without open surgery because of advanced disease or significant comorbid factors. This group was significantly older, with a mean age of 73 years.
In the Nagoya cohort, the overall resectability rate was 79%; 122 of 155 patients underwent surgical resection (Table 1). The mean age of the resection group was 60 years. Four patients in this group had limited metastatic disease found during surgery. Palliative resections were performed in these four patients because they were young and had good performance status. Ten patients (6%) underwent surgical exploration, but their cancers were found to be unresectable at surgery. Six patients in this group had metastatic disease and four had locally advanced disease. The mean age for this group who underwent surgical exploration but had unresectable disease was 64 years. Twenty-three patients (15%) underwent percutaneous transhepatic biliary drainage (PTBD) without open surgery because of advanced disease or significant comorbid factors. This group had a mean age of 64 years.
Extent of Resection and Resection Margins
Significant differences were noted in the extent of resection and margin-negative resectability between the Lahey and Nagoya cohorts (Table 2).
Table 2. TYPES OF SURGICAL RESECTION
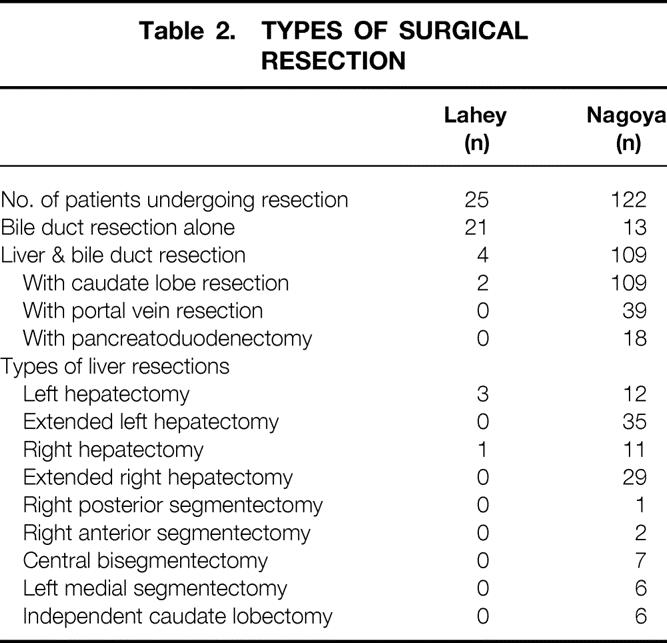
En bloc caudate lobectomy was performed in 8% of the Lahey patients who underwent resection and 89% of the Nagoya patients who underwent resection.
Among the 25 Lahey patients who underwent resection, 7 (28%) had margin-negative resections, 10 (40%) had infiltrative cancer at the proximal margin, 6 (24%) had infiltrative cancer at both proximal and distal margins, and 2 (8%) had carcinoma in situ at the distal margin. In the subgroup of 21 patients undergoing bile duct resection alone, 16 (76%) had positive resection margins. In the subgroup of four patients undergoing combined liver and bile duct resection, two patients (50%) had positive resection margins.
Among the 122 Nagoya patients who underwent resection, 96 (79%) had margin-negative resections, 10 (8%) had infiltrative cancer at the proximal margin, 2 (2%) had infiltrative cancer at both proximal and distal margins, 5 (4%) had carcinoma in situ at one or both resection margins, and 9 (7%) had cancer around the hepatic artery margin. In the subgroup of 13 patients who had bile duct resection alone, 6 (46%) had positive resection margins. In the subgroup of 109 patients undergoing combined liver and bile duct resection, 20 (18%) had positive resection margins.
Of all the patients who underwent surgical exploration, margin-negative resection was achieved in 7 of 78 patients (9%) in the Lahey cohort and in 96 of 132 patients (73%) in the Nagoya cohort. In both cohorts, combined liver and bile duct resection resulted in a higher rate of margin-negative resections compared with bile duct resection alone.
Treatment Outcome
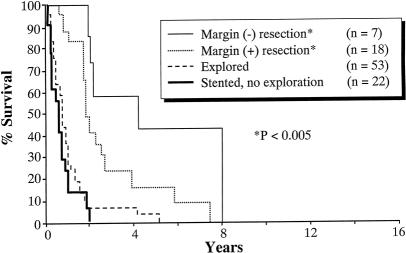
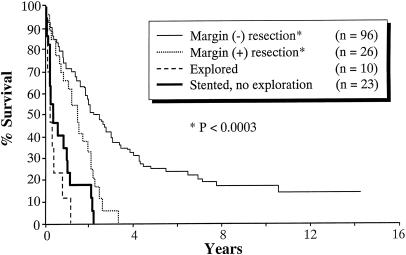
Significant differences were observed between the Lahey and Nagoya cohorts in the overall 5- and 10-year survival rates. The overall 5-year survival rate was 7% for the 100 patients treated at the Lahey Clinic and 16% for the 155 patients treated at Nagoya University (P = .034). The overall 10-year survival rate was 0% for the Lahey cohort and 12% for the Nagoya cohort (P < .0001). Survival analyses stratified by resectability and resection margin status are shown for Lahey patients (Fig. 1) and for Nagoya patients (Fig. 2).
Figure 1. Kaplan-Meier survival analysis for Lahey patients (n = 100).
Figure 2. Kaplan-Meier survival analysis for Nagoya patients (n = 155).
In the Lahey patients with negative resection margins (n = 7), the 5- and 10-year survival rates were 43% and 0%, respectively. The median survival of this group was 59 months. This was significantly longer than that of the patients who did not undergo resection (P = .0000) and the margin-positive patients (P < .05). In the Nagoya patients with negative resection margins (n = 96), the 5- and 10-year survival rates were 24% and 18%, respectively. The median survival for this group was 27 months. This was significantly longer than that of the patients who did not undergo resection (P < .00001) and the margin-positive patients (P < .0003).
Further stratification of the Nagoya cohort by whether portal vein resection was needed to achieve negative resection margins showed that for patients not needing portal vein resection, the 5- and 10-year survival rates were 35% and 26%, respectively. Only 7% of the patients needing portal vein resection lived 5 years, and none survived 10 years. The median survival for patients not needing portal vein resection was 48 months, in contrast to 20 months for patients needing portal vein resection.
In the Lahey patients with positive resection margins (n = 18), the 5-year survival rate was 15%; none survived 10 years. The median survival for this subgroup was 31 months. The longest survivor in this group had carcinoma in situ in the distal margin; he lived 7.5 years but died of carcinomatosis. In the Nagoya patients with positive resection margins (n = 26), none of the patients survived 5 years. The median survival was 18 months. The longest survivor lived 3.5 years.
The 5-year survival rate was 2% among the 53 Lahey patients who underwent surgical exploration but whose cancers were deemed unresectable. The median survival for this group was 11 months. The one 5-year survivor had a good quality of life with an external biliary drainage catheter until she died of disease progression and liver failure 5 years after diagnosis. The worst outcome in the Nagoya cohort occurred in the group of 10 patients who underwent surgical exploration but whose cancers were unresectable. The median survival for this group was 3 months, and none survived 2 years.
Survival rates were similar in patients who underwent biliary stenting without surgical exploration at Lahey (n = 22) and at Nagoya (n = 23). The median survival was 8 months for the Lahey cohort and 5 months for the Nagoya cohort.
Survival Outcome in Patients With Nodal Metastases
Among the 122 patients who underwent resection at Nagoya University Hospital, 56 (46%) had histologically proven lymph node metastases. The 3-, 5-, and 10-year survival rates for these 56 patients were 20%, 10%, and 6%, respectively (Fig. 3). The median survival for this subgroup was 20 months. Although the survival of this group of node-positive patients was significantly shorter than the node-negative group, it was significantly longer than the group who did not undergo resection.
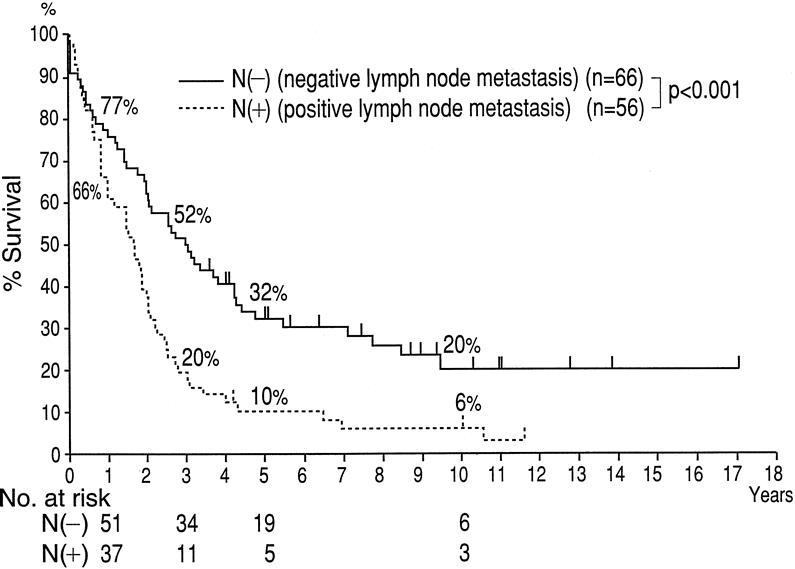
Figure 3. Kaplan-Meier survival analysis stratified by nodal involvement for Nagoya patients (n = 122).
Among the 25 patients who underwent resection at the Lahey Clinic, 3 had histologically proven lymph node metastases. The 2-year survival rate was 33%, and the 3-year survival rate was 0%. The median survival for these three patients was 23 months. The small number of patients with metastatic lymph nodes who underwent resection at Lahey precluded meaningful statistical analysis for this subgroup.
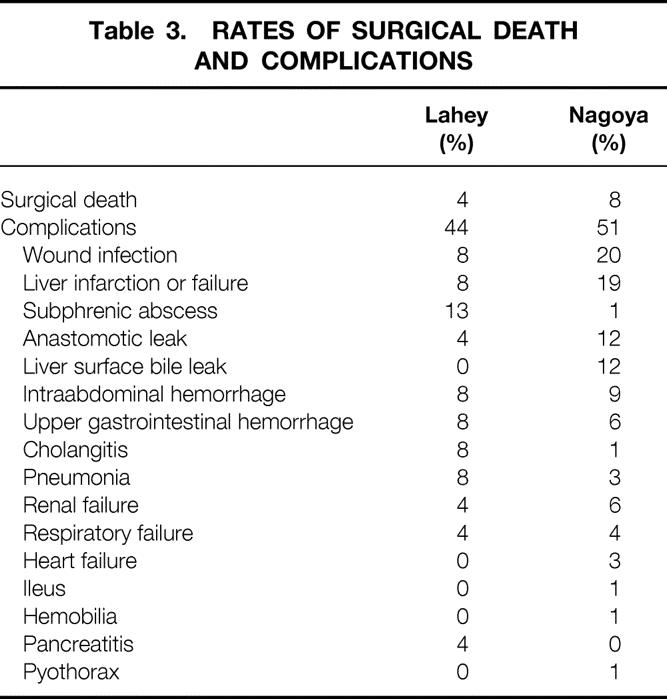
Surgical Death and Complications
The rates of surgical death and complications of the two patient cohorts were similar (P = .306 and P = .295, respectively) (Table 3). For both cohorts, the cause of surgical death was liver failure. No significant associated increase in death and complications was observed in the Nagoya cohort, even though more combined liver and bile duct resections were performed.
Table 3. RATES OF SURGICAL DEATH AND COMPLICATIONS
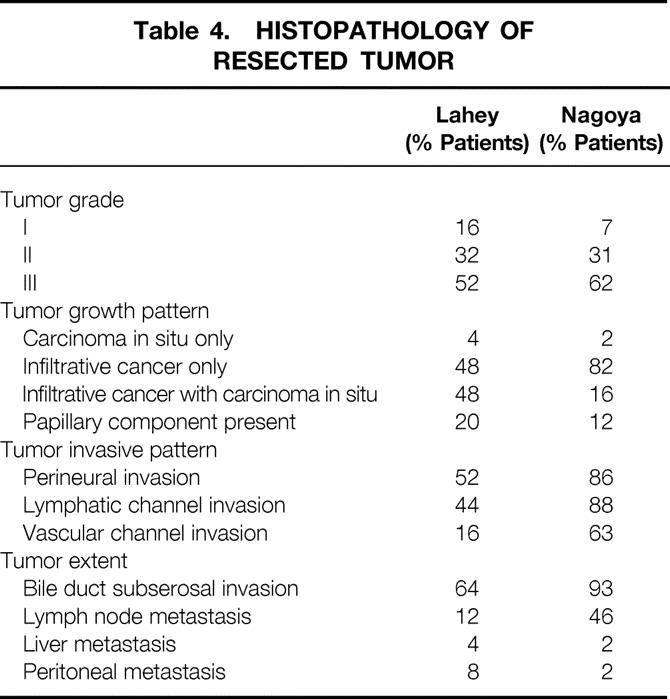
Histopathology of Resected Tumors
Review of the exchanged histologic slides (13 Lahey cases, 20 Nagoya cases) resulted in 100% concordance between our institutions in the diagnostic criteria for invasive and in situ adenocarcinoma of the bile duct. Results of histopathologic analysis are compared in Table 4. Most of the resected tumors in both cohorts were diffusely infiltrative. The incidence of perineural invasion, 17 lymphatic or vascular channel invasion, lymph node metastasis, and bile duct subserosal invasion was higher in the Nagoya cohort, indicating an overall higher-stage disease. The higher frequency of carcinoma in situ observed in Lahey patients may reflect earlier (lower-stage) disease in this cohort, because one might predict that advanced invasive cancer is more likely to destroy or overgrow precursor in situ lesions. Twenty percent of the Lahey cohort who underwent resection and 12% of the Nagoya cohort who underwent resection had papillary intraductal histology with or without invasion, known for its more favorable natural history. 18
Table 4. HISTOPATHOLOGY OF RESECTED TUMOR
Preoperative Diagnostics and Interventions
Of the 25 Lahey patients who underwent resection, 77% had preoperative endoscopic retrograde cholangiography with or without stenting. Because this diagnostic study frequently did not show the hilar ductal anatomy in enough detail to determine resectability, 64% of the patients underwent preoperative percutaneous transhepatic cholangiography with or without biliary drainage. Preoperative selective celiac and mesenteric arteriography with portal venography was obtained in 18% of the patients. Preoperative ultrasound or computed tomography was performed in all patients.
Extensive preoperative staging investigations and interventions 19 were performed in the 122 Nagoya patients who underwent resection. Ninety-five percent underwent PTBD with selective cholangiography. The number of PTBD catheters inserted was dictated by the need for adequate visualization and drainage of all occluded segmental biliary radicals. 20,21 The Nagoya approach to PTBD catheter placement differs from the American approach in that the distal tip of the catheter remains in the mid-common bile duct and does not traverse the sphincter of Oddi. The theoretical advantage of not having the distal catheter tip sit in the duodenum is reduction of enteric contamination of the biliary tree and cholangitis. Biliary drainage was continued for 3 to 6 weeks, aiming at a return to normal of the serum bilirubin level and hepatic synthetic function. Patients with persistent hyperbilirubinemia or cholangitis 21 after an appropriate period of PTBD were excluded from major liver resection because of the prohibitive risk of postoperative liver failure. 22,23 Fifty percent of the Nagoya patients who underwent resection underwent percutaneous transhepatic cholangioscopy through the PTBD tract. Percutaneous transhepatic cholangioscopy was performed on patients suspected of having tumors with superficial spreading or multifocal patterns. Biopsy under direct visualization of the cholangioscope permitted precise mapping of the extent of cancer in the biliary tree. 24 Dynamic computed tomography, selective visceral arteriography, and percutaneous transhepatic portal venography were obtained on all surgical patients. Preoperative portal vein embolization was performed in patients who needed extensive liver resection. Preoperative portal vein embolization has been shown to decrease the incidence of postoperative liver failure 25 by inducing compensatory hypertrophy of the future remnant liver.
Postoperative Management of Biliary Stents
Transhepatic biliary stents were placed across the anastomosis or anastomoses for biliary decompression in all patients who underwent resection at both institutions. At Nagoya University Hospital, a cholangiogram was obtained in the second postoperative week to confirm the patency and integrity of the anastomoses and to define the exact segmental location of the anastomoses. The catheters were removed in the third postoperative week. At the Lahey Clinic, a cholangiogram was obtained in the third postoperative week and the catheters were removed between the fourth and sixth postoperative week.
Adjuvant Chemotherapy or Radiation
At Nagoya University Hospital, none of the patients who underwent resection received adjuvant chemotherapy or radiation. At the Lahey Clinic, none of the patients who underwent resection received adjuvant chemotherapy; five patients with positive resection margins and one patient with negative resection margins had adjuvant brachytherapy or external radiation. The median survival for these six patients who underwent resection and were given adjuvant radiation was 22.5 months (range 21–46 months), showing no improvement in survival compared with similar patients without adjuvant radiation. Because of the small number of patients receiving adjuvant radiation, no conclusions regarding the role of adjuvant radiation can be drawn from our study.
DISCUSSION
In this study, which compares the experience of one American and one Japanese medical center, we noted significant differences in our guidelines for resectability, the scope of preoperative staging, the extent of resection, the margin-negative resection rates, and the long-term survival outcome. Similarities were observed in the number of patients treated per year during the study period, patient demographics, tumor type, and surgical expertise.
The patients who underwent surgical exploration but had unresectable disease made up the largest segment (53%) of the Lahey cohort, compared with 6% of the Nagoya cohort. Margin-negative resections were achieved in 28% of the Lahey cohort who underwent resection compared with 79% of the Nagoya cohort who underwent resection. In the Lahey cohort, the proximal bile duct margin was especially problematic: it was positive in 40% of the patients who underwent resection, compared with only 8% of the Nagoya cohort. Combined bile duct and liver resection with caudate lobectomy was performed in 8% of the patients who underwent resection at Lahey versus 89% at Nagoya.
The differences in resectability criteria and radicality of resection between our two institutions were reflected in the differences observed in specimen histopathology (Table 4). The specimens from the Nagoya cohort showed higher-stage disease, with more adverse histologic features, than those from the Lahey cohort. Despite this, a better survival rate was observed in the Nagoya cohort.
Resection in patients with nodal metastases yielded several long-term survivors in the Nagoya cohort (Fig. 3). By the Lahey resectability guidelines, these patients probably would have been in the unresectable group and would not have had a chance for long-term survival. If resection with lymphadenectomy could render a patient grossly disease-free, we recommend its use in patients with good performance status. We caution that the expertise of the surgical team is pivotal to the success of this aggressive resection approach. Heroic resection cannot be justified if it is associated with a significantly increased rate of surgical complications. The extensive resection undertaken at Nagoya University requires an average surgical time of 10 to 12 hours. Our Nagoya colleagues use a team approach to this technically challenging dissection. The surgical team is headed by two senior surgeons. The chief surgeon (Y.N.) is assisted by a senior surgeon, not a surgical trainee, during the most exacting portion of the procedure—the resection of the tumor, lymph nodes, involved vasculature, and vascular reconstruction. The last stage of the procedure, the biliary reconstruction, is completed by one of the senior surgeons or a fresh team of surgeons.
We propose three postulates for the observed differences between our institutions in the management of hilar cholangiocarcinoma:
Before each patient’s planned surgery, our Nagoya colleagues decided on the radicality needed to achieve margin-negative resections based on a systematic approach to preoperative staging. An extensive array of cholangiographic and angiographic studies defined the exact cancer location and extension within and outside the biliary tree.
Our Nagoya colleagues had more liberal resectability criteria. In locally advanced disease, they performed combined caudate lobe resection, lymphadenectomy, resection of hilar vasculature, or even resection of adjacent duodenum and pancreas.
Familiarity with caudate lobe anatomy and experience with resection of the caudate lobe 26,27 permitted our Nagoya colleagues to remove tumors involving the caudate lobe safely; these tumors were often deemed unresectable at Lahey.
Mizumoto et al 28 in 1986 cautioned that failure to achieve tumor clearance in the caudate lobe is responsible for some tumor recurrences after resection of hilar cholangiocarcinoma. This contention was confirmed by an autopsy study by Gazzaniga et al 9 in 1993: 4 of their 19 patients (21%) had recurrence in the caudate lobe after resection. In an elegant three-dimensional tumor mapping study, Suzuki et al 29 in 1989 demonstrated cancer invasion of the caudate bile duct epithelium in 10 of 10 resected specimens. Additional studies of resected specimens by Nimura et al 14 in 1990, Ogura et al 15 in 1993, and Sugiura et al 16 in 1994 showed an incidence of caudate bile duct invasion by hilar cholangiocarcinoma of 31% to 98%. For this reason, we recommend en bloc caudate lobe resection when the hepatic ductal confluence is encompassed by hilar cholangiocarcinoma. This concept is now embraced not only by Japanese surgeons but also by Western surgeons. 4,8,30
Resection of the portal vein and hepatic artery in patients with locally advanced hilar cholangiocarcinoma remains controversial. Technically, it is possible to carry out excision of involved portal vein at its bifurcation or in the main portal trunk with subsequent end-to-end anastomosis or vein graft. 31 Arterial reconstruction is a more challenging problem but can be done using microsurgical techniques under magnification. Likewise, hepatic resection combined with pancreatoduodenectomy is technically possible 32–34 but remains controversial in terms of the risk of surgery versus the survival benefit to the patient.
Traditional surgical teaching espouses that full surgical exploration will reveal the extent of tumor. However, in the setting of the extensive sclerosis often seen with hilar cholangiocarcinoma, it is difficult during surgery to determine the extent of biliary and vascular involvement. Such dissection is exacting for the surgeon and risky to the patient when done without preoperative high-quality cholangiographic and angiographic studies to serve as road maps. Other authors 9,30 have voiced the same caution.
In his review of hilar cholangiocarcinoma, Boerma 35 argued that the slightly increased survival associated with combined liver resection is not justified by the tradeoff in surgical deaths. From our comparative study, we arrived at a different conclusion. Other reports 3–10,13,15,36 also have shown that combined bile duct and liver resection for hilar cholangiocarcinoma does not significantly raise the rates of surgical complications and death in the hands of an experienced hepatobiliary surgical team.
This comparative study of an American and a Japanese patient cohort clarified and reinforced several issues in the management of hilar cholangiocarcinoma:
Regardless of ethnicity, patients who had undergone resection with negative margins had the most favorable prognosis.
Improved survival is a function of margin-negative resection and not a function of the magnitude of resection. 36–39
A higher rate of margin-negative resections can be achieved with the appropriate use of combined bile duct and liver resection with caudate lobectomy. 40
In centers with expertise in hepatobiliary surgery, combined bile duct and liver resection does not result in an increased rate of surgical death compared with bile duct resection alone.
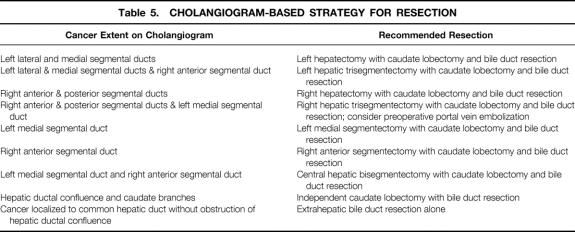
Detailed preoperative biliary and angiographic studies are essential to guide the extent of resection. We recommend the cholangiogram-based surgical strategy shown in Table 5.
Table 5. CHOLANGIOGRAM-BASED STRATEGY FOR RESECTION
Footnotes
Correspondence: Yuji Nimura, MD, First Dept. of Surgery, Nagoya University School of Medicine, 65 Tsurumaicho, Showaku, Nagoya, 466-8550, Japan.
Dr. Tsao is currently with the Dept. of Surgery, New England Medical Center, Boston, MA.
Dr. Rossi is currently with Catholic University, Santiago, Chile.
Accepted for publication February 28, 2000.
References
- 1.Langer JC, Langer B, Taylor BR, et al. Carcinoma of the extrahepatic bile ducts: results of an aggressive surgical approach. Surgery 1985; 98: 752–759. [PubMed] [Google Scholar]
- 2.Pinson CW, Rossi RL. Extended right hepatic lobectomy, left hepatic lobectomy, and skeletonization resection for proximal bile duct cancer. World J Surg 1988; 12: 52–59. [DOI] [PubMed] [Google Scholar]
- 3.Pichlmayr R, Ringe B, Lauchart W, et al. Radical resection and liver grafting as the two main components of surgical strategy in the treatment of proximal bile duct cancer. World J Surg 1988; 12: 68–77. [DOI] [PubMed] [Google Scholar]
- 4.Fortner JG, Vitelli CE, Maclean BJ. Proximal extrahepatic bile duct tumors. Analysis of a series of 52 consecutive patients treated over a period of 13 years. Arch Surg 1989; 124: 1275–1279. [DOI] [PubMed] [Google Scholar]
- 5.Cameron JL, Pitt HA, Zinner MJ, et al. Management of proximal cholangiocarcinomas by surgical resection and radiotherapy. Am J Surg 1990; 159: 91–98. [DOI] [PubMed] [Google Scholar]
- 6.Hadjis NS, Blenkharn JI, Alexander N, et al. Outcome of radical surgery in hilar cholangiocarcinoma. Surgery 1990; 107: 597–604. [PubMed] [Google Scholar]
- 7.Reding R, Buard J, Lebeau G, Launois B. Surgical management of 552 carcinomas of the extrahepatic bile ducts. Results of the French Surgical Association survey. Ann Surg 1991; 213: 236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg 1992; 215: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gazzaniga GM, Ciferri E, Bagarolo C, et al. Primitive hepatic hilum neoplasm. J Surg Oncol 1993; 3 (Suppl): 140–146. [DOI] [PubMed] [Google Scholar]
- 10.Nagorney DM, Donohue JH, Farnell MB, et al. Outcomes after curative resections of cholangiocarcinoma. Arch Surg 1993; 128: 871–879. [DOI] [PubMed] [Google Scholar]
- 11.Baer HU, Stain SC, Dennison AR, et al. Improvements in survival by aggressive resections of hilar cholangiocarcinoma. Ann Surg 1993; 217: 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Washburn WK, Lewis WD, Jenkins RL. Aggressive surgical resection for cholangiocarcinoma. Arch Surg 1995; 130: 270–276. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki Y, Okamura T, Ozaki A, et al. Surgical treatment for carcinoma at the confluence of the major hepatic ducts. Surg Gynecol Obstet 1986; 162: 457–464. [PubMed] [Google Scholar]
- 14.Nimura Y, Hayakawa N, Kamiya J, Kondo S, Shionoya S. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg 1990; 14: 535–544. [DOI] [PubMed] [Google Scholar]
- 15.Ogura Y, Mizumoto R, Tabata M, et al. Surgical treatment of carcinoma of the hepatic duct confluence: analysis of 55 resected carcinomas. World J Surg 1993; 17: 85–92. [DOI] [PubMed] [Google Scholar]
- 16.Sugiura Y, Nakamura S, Iida S, et al. Extensive resection of the bile ducts combined with liver resection for cancer of the main hepatic duct junction: a cooperative study of the Keio Bile Duct Cancer Study Group. Surgery 1994; 115: 445–451. [PubMed] [Google Scholar]
- 17.Bhuiya MMR, Nimura Y, Kamiya J, et al. Clinicopathological studies on perineural invasion of bile duct carcinoma. Ann Surg 1992; 215: 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakamoto E, Nimura Y, Hayakawa N, et al. The pattern of infiltration at the proximal border of hilar bile duct carcinoma. A histologic analysis of 62 resected cases. Ann Surg 1998; 227: 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagino M, Nimura Y, Kamiya J, et al. Preoperative management of hilar cholangiocarcinoma. J Hepatobil Pancreat Surg 1995; 2: 215–223. [Google Scholar]
- 20.Nagino M, Hayakawa N, Nimura Y, et al. Percutaneous transhepatic biliary drainage in patients with malignant biliary obstruction of the hepatic confluence. Hepato-Gastroenterology 1992; 39: 296–300. [PubMed] [Google Scholar]
- 21.Nimura Y, Kamiya J, Kondo S, et al. Technique of inserting multiple biliary drains and management. Hepato-Gastroenterology 1995; 42: 323–331. [PubMed] [Google Scholar]
- 22.Kanai M, Nimura Y, Kamiya J, et al. Preoperative intrahepatic segmental cholangitis in patients with advanced carcinoma involving the hepatic hilus. Surgery 1996; 119: 498–504. [DOI] [PubMed] [Google Scholar]
- 23.Miyagawa S, Makuuchi M, Kawasaki S. Outcome of extended right hepatectomy after biliary drainage in hilar bile duct cancer. Arch Surg 1995; 130: 759–763. [DOI] [PubMed] [Google Scholar]
- 24.Nimura Y. Staging of biliary carcinoma: cholangiography and cholangioscopy. Endoscopy 1993; 25: 76–80. [DOI] [PubMed] [Google Scholar]
- 25.Nagino M, Nimura Y, Kamiya J, et al. Right or left trisegment portal vein embolization before hepatic trisegmentectomy for hilar bile duct carcinoma. Surgery 1995; 117: 677–681. [DOI] [PubMed] [Google Scholar]
- 26.Kamiya J, Nimura Y, Hayakawa N, et al. Preoperative cholangiography of the caudate lobe: surgical anatomy and staging for biliary carcinoma. J Hepatobil Pancreat Surg 1994; 4: 385–389. [Google Scholar]
- 27.Nimura Y, Hayakawa N, Kamiya J, et al. Hilar cholangiocarcinoma—surgical anatomy and curative resection. J Hepatobil Pancreat Surg 1995; 2: 239–248. [Google Scholar]
- 28.Mizumoto R, Kawarada Y, Suzuki H. Surgical treatment of hilar carcinoma of the bile duct. Surg Gynecol Obstet 1986; 162: 153–162. [PubMed] [Google Scholar]
- 29.Suzuki M, Takahashi T, Ouchi K, et al. The development and extension of hepatohilar bile duct carcinoma. A three-dimensional tumor mapping in the intrahepatic biliary tree visualized with the aid of a graphic computer system. Cancer 1989; 64: 658–666. [DOI] [PubMed] [Google Scholar]
- 30.Lygidakis NJ, van der Heyde MN, Houthoff HJ. Surgical approaches to the management of primary biliary cholangiocarcinoma of the porta hepatis: the decision-making dilemma. Hepato-Gastroenterology 1988; 35: 261–267. [PubMed] [Google Scholar]
- 31.Nimura Y, Hayakawa N, Kamiya J, et al. Combined portal vein and liver resection for carcinoma of the biliary tract. Br J Surg 1991; 78: 727–731. [DOI] [PubMed] [Google Scholar]
- 32.Nimura Y, Hayakawa N, Kamiya J, et al. Hepatopancreatoduodenectomy for advanced carcinoma of the biliary tract. Hepato-Gastroenterology 1991; 3: 170–175. [PubMed] [Google Scholar]
- 33.Tsukada T, Yoshida K, Aono T, et al. Major hepatectomy and pancreatoduodenectomy for advanced carcinoma of the biliary tract. Br J Surg 1994; 81: 108–110. [DOI] [PubMed] [Google Scholar]
- 34.Miyagawa S, Makuuchi M, Kawasaki S, et al. Outcome of major hepatectomy with pancreatoduodenectomy for advanced biliary malignancies. World J Surg 1996; 20: 77–80. [DOI] [PubMed] [Google Scholar]
- 35.Boerma EJ. Research into the results of resection of hilar bile duct cancer. Surgery 1990; 108: 572–580. [PubMed] [Google Scholar]
- 36.Pichlmayr R, Weimann A, Klempnauer J, et al. Surgical treatment in proximal bile duct cancer: single-center experience. Ann Surg 1996; 224: 628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su CH, Tsay SH, Wu CC, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg 1996; 223: 384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyazaki M, Ito H, Nakagawa K, et al. Aggressive surgical approaches to hilar cholangiocarcinoma. Hepatic or local resection? Surgery 1998; 123: 131–136. [PubMed] [Google Scholar]
- 39.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996; 224: 463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burke EC, Jarnagin WR, Hachwald SN, et al. Hilar cholangiocarcinoma. Pattern of spread, the importance of hepatic resection for curative operation and a presurgical clinical staging system. Ann Surg 1998; 228: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]