Abstract
Objective
To discuss the pathophysiology and incidence of the short esophagus, to review the history of treatment, and to describe diagnosis and possible treatments in the era of laparoscopic surgery.
Summary Background Data
The entity of the short esophagus in antireflux surgery is seldom discussed in the laparoscopic literature, despite its emphasis in the open literature for more than 40 years. This may imply that many laparoscopic patients with short esophagi are unrecognized and perhaps treated inappropriately. Intrinsic shortening of the esophagus most commonly occurs in patients with chronic gastroesophageal reflux disease that involves recurring cycles of inflammation and healing, with subsequent fibrosis. The actual incidence of the short esophagus is estimated to be approximately 10% of patients undergoing antireflux surgery. Of this group, 7% can be appropriately managed with extensive mediastinal mobilization of the esophagus to achieve the required esophageal length. The remaining 3% require an aggressive surgical approach, including the use of gastroplasty procedures, to create an adequate length of intraabdominal esophagus to perform a wrap. Several effective minimally invasive techniques have been developed to deal with the short esophagus.
Conclusions
Because a short esophagus is uncommon, there is a natural concern that many surgeons will not perform enough antireflux procedures to become familiar with its diagnosis and management. A complete understanding of the short esophagus and methods for surgical correction are critical to avoid “slipped” wraps and mediastinal herniation and to achieve the best patient outcome.
The relation between the short esophagus and antireflux surgery has been a topic of keen interest in the esophageal literature of the past 40 years. 1–7 The result of this interest has been the generation of a wealth of data regarding its pathophysiology and treatment. There is, however, a striking paucity of reference to the short esophagus in the current laparoscopic literature. This is worrisome because it may imply that many patients undergoing laparoscopic surgery who have a short esophagus are unrecognized and perhaps treated inappropriately. This may also explain the higher failure rates and increased postoperative dysphagia reported by some authors. 8
Decades of experience with open fundoplications have established certain principles and surgical techniques as essential for successful surgical outcomes. These concepts include thorough preoperative testing, routine division of the short gastric vessels, crural closure, and repairs performed without tension around a 2.5- to 3-cm length of intraabdominal esophagus. 9–13 Such principles are no less important in laparoscopic surgery to ensure excellent results. 14 The defining aspect of a tension-free hiatal hernia repair is the proper treatment of an intrinsically shortened esophagus. When such a short esophagus is not recognized and treated, the risk of a “slipped” or misplaced fundoplication or a crural disruption with subsequent herniation of the wrap into the mediastinum is increased (Fig. 1). 15 This occurrence is thought to be responsible for 20% to 33% of the surgical failures after open or laparoscopic fundoplication. 16–19 The reoperative surgery that is required to correct such failures is known to have a higher rate of surgical complications and a less favorable long-term functional result. 16,20
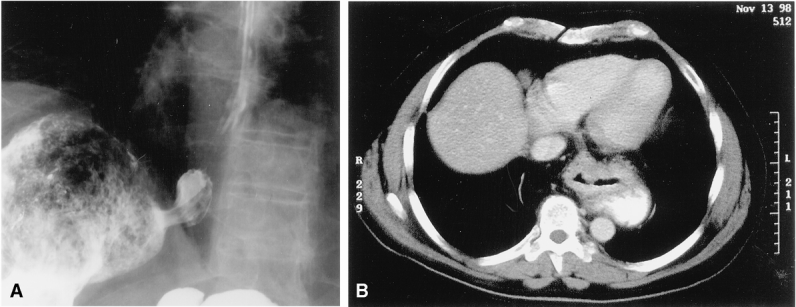
Figure 1. (A) Barium esophagram showing a “slipped” or misplaced Nissen. (B) Computed tomography scan showing a herniated fundoplication resulting from a wrap performed under tension.
The purpose of this review is to discuss the pathophysiology and incidence of the short esophagus, to review the history of treatment for the condition, and to describe its diagnosis and treatment in the era of laparoscopic surgery.
PATHOPHYSIOLOGY
Intrinsic shortening of the esophagus results most commonly from the chronic inflammation that accompanies gastroesophageal reflux disease (GERD). 21 GERD primarily occurs as a result of a dysfunction of the lower esophageal sphincter (LES) that allows either acid or alkaline contents to regurgitate up into the esophagus. Because the squamous epithelium of the esophageal mucosa is not an effective barrier to refluxed juices, a “burn” to the esophagus results, similar to that occurring in the case of ingested corrosive chemicals. An inflammatory response ensues, with the inevitable stages of edema, inflammatory cell infiltration, subsequent healing, and eventual fibrosis. This process eventually involves the deeper muscular layers of the esophageal wall and may even extend transmurally into the periesophageal tissues of the mediastinum. With repeated cycles of injury and repair over time, functional and irreversible damage occurs to the involved esophagus. Contraction of the collagen in the transmural fibrous scar can occur circumferentially, producing a peptic stricture, or longitudinally, resulting in a short esophagus.
Although few would deny this known physiologic response to injury, occasional surgeons argue against the existence of an intrinsically shortened esophagus. 22–24 Such thinking contradicts the preponderance of evidence from pathologic specimens, data from animal models, known responses of tissues to burns with contracture formation, and the incidence of surgical failures after fundoplication surgery. 2,9 Other entities associated with esophageal shortening include type III paraesophageal hernias, sarcoidosis, Barrett’s metaplasia, caustic ingestion, scleroderma, and Crohn’s disease, all of which can result in a profound inflammatory reaction with significant cephalad displacement of the gastroesophageal junction (GEJ).
THE TERM “SHORT ESOPHAGUS”
Most esophagi that are short based on preoperative imaging are actually of normal length. It is therefore helpful to think of the short esophagus, as evaluated in the operating room, as falling into three categories (Fig. 2): a true, nonreducible short esophagus; a true but reducible short esophagus; and an apparent short esophagus. Perioperative endoscopic or radiologic studies document that all three groups have a GEJ located at or above the hiatus. Both the true reducible and nonreducible short esophagi have sustained enough chronic damage to the esophagus to lead to actual intrinsic shortening, whereas patients with an apparent short esophagus have a normal-length esophagus that is merely accordioned into the distal mediastinum. The only way to differentiate between these types is surgical mobilization of the mediastinal esophagus. In most patients (i.e., true, reducible short esophagus and apparent short esophagus), it is possible to reduce the GEJ to at least 2.5 cm below the hiatus. However, in a few patients (i.e., true, nonreducible short esophagus), intraabdominal reduction cannot be accomplished despite extensive transmediastinal or transthoracic esophageal mobilization.
Figure 2. The true (A) and apparent (B) shortened esophagus. The apparent shortened esophagus is accordioned onto itself in the distal mediastinum and can be easily reduced. Most true, shortened esophagi can be reduced with extensive mediastinal dissection. However, a few shortened esophagi are nonreducible and require a gastroplasty.
INCIDENCE
The precise incidence of the truly shortened esophagus is unknown. In a review of the open and laparoscopic literature, the frequency of esophageal shortening ranges widely from the 60% reported by Pearson and Todd 25 to 0% reported by Hill et al 23 and some laparoscopic series. 22 In fact, at one point, Pearson and Todd described all patients undergoing an antireflux procedure as needing a Collis-Nissen. 25 A problem with the series reporting extremes of incidence is that none describes individual patient assessment using a clear definition of a short esophagus (namely, a GEJ that cannot be surgically mobilized to lie >2.5 cm below the hiatus without tension). In the open literature, where a lengthening procedure was performed based on assessed need, 8% to 10% of all patients undergoing fundoplication for GERD were reported to require an esophageal lengthening procedure, consisting of either extensive mediastinal dissection (type II dissection) or a Collis gastroplasty. 26,27 Two large consecutive patient series in the laparoscopic literature found similar incidences of esophageal shortening. Both studies described a 3% to 4% incidence of patients requiring a Collis gastroplasty. 28,29 One of the reports included 500 patients, 7% of whom required a type II dissection and 3% of whom required a Collis, for a total short esophagus incidence of 10%. 28 These statistics seem to correlate with the incidence in the open literature.
The incidence reported in the literature might be higher than that encountered in a routine surgical practice for two reasons. First, many reports in the literature originate from tertiary referral units and specialty centers, which would be expected to see more large hernias, redo surgeries, and severe, complicated disease, all of which are associated with higher rates of esophageal shortening. Also, many reports, particularly of open series, are based on data collected in the past. It may be that the widespread use of proton pump inhibitors and earlier referral for minimally invasive treatments have decreased the current incidence of severe, complicated disease including shortening.
DIAGNOSIS AND EVALUATION
Preoperative findings that should raise the index of suspicion for a short esophagus are outlined in Table 1. 2,25,30 These indicators are not highly specific; in particular, both esophagogastroduodenoscopy (EGD) findings and barium studies are subject to performer and reviewer bias. In a masked review of 15 patients who required a Collis gastroplasty for esophageal lengthening, the positive predictive value of the preoperative barium esophagram was only 50%. 31 In general, preoperative examinations serve only to increase the clinician’s index of suspicion. Most would agree that the actual diagnosis of a short esophagus can be made only in the operating room. A tension-free, 2.5- to 3-cm length of intraabdominal esophagus is required for proper placement of a wrap (Fig. 3). If 2.5 cm of intraabdominal esophagus is not present after a standard dissection and using minimal traction, the patient has a short esophagus that must be addressed.
Table 1. PREOPERATIVE INDICATORS FOR THE PRESENCE OF A SHORT ESOPHAGUS
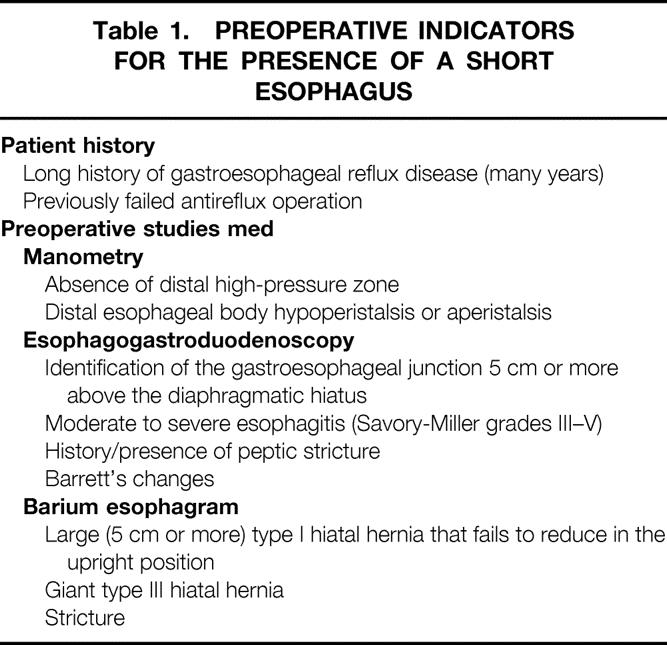

Figure 3. Intraoperative assessment of esophageal length. The opened width of a standard dissector is 2.5 cm (inset). Using the opened instrument as a gauge, the length of intraabdominal esophagus can be ascertained. If there is confusion about the location of the gastroesophageal junction, intraoperative endoscopy should be used.
An additional consideration is the evaluation and treatment of patients with failed previous antireflux surgery. Fundoplications can fail because of wrap herniation, slippage, or disruption; all may be due to failure to recognize a short esophagus at the initial surgery. The workup of patients with a failed fundoplication should include upper endoscopy to assess the wrap and to determine the position of the LES, a barium swallow to delineate herniation and to define the anatomy, esophageal motility to assess body function and to measure the wrap function, and 24-hour pH studies for symptom correlation (see Table 1). Any of these evaluations can suggest the diagnosis of a short esophagus, which should be confirmed and treated at the time of revisionary surgery.
TREATMENT OPTIONS
The clinical entity of the short esophagus was first described more than 40 years ago. Several surgical options for treating the short esophagus have been reported, including esophagectomy, intrathoracic fundoplication, and lengthening procedures (Table 2). It is an inappropriate but frequently used technique to place aggressive axial traction on the stomach without adequately mobilizing the mediastinal esophagus. This can result in a deceptive elongation of the proximal stomach, which can be inaccurately identified as the distal esophagus and wrapped. The result is often referred to as a “slipped” Nissen but is probably more accurately labeled a “misplaced” Nissen (see Fig. 1). 15 A misplaced wrap can be problematic, with severe postoperative dysphagia and late dilatation of the gastric pouch above the distal high-pressure zone. It is also wrong to perform a fundoplication properly around the distal esophagus, when the GEJ is retracted below the diaphragm under tension. As a violation of one of the basic tenets of surgery, such a repair will inevitably be subject to high rates of dehiscence or transdiaphragmatic herniation.
Table 2. TREATMENT OPTIONS

Hill Esophagopexy
Hill 23 has long recommended esophagogastropexy (Hill procedure) as a treatment for all patients with GERD, including those with a short esophagus. The good results described by Hill advocates for patients having a foreshortened esophagus may be related to the fact that the procedure does not need to achieve a length of intraabdominal esophagus. Unfortunately, the Hill procedure has never obtained widespread acceptance because of its perceived complexity.
Intrathoracic Fundoplication
One proposed solution to the problem of a short esophagus has been the intrathoracic fundoplication. Good control of reflux has been documented with this approach. 32–34 Along with reflux control, however, there are often substantial complications associated with this iatrogenically created herniated wrap, including epigastric or chest pain, dysphagia, and even strangulation, perforation, ulceration, or bleeding. 32–37 Because of these complications, intrathoracic fundoplication is seldom if ever recommended. 35–39
Esophagectomy
Occasionally, total esophagectomy and reconstruction may be the best option for the severely damaged esophagus. A small study of a short esophagus population from DeMeester et al 40 recently documented a better symptomatic and functional outcome of esophagectomy with colon interposition compared with a Collis-Belsey procedure. Obviously, the significant complication rate of esophagectomy is a major concern with this option, and to date it is usually reserved for extremely severe or recalcitrant cases.
Esophageal Lengthening Procedures
Techniques to lengthen the esophagus surgically have been described for some time. A circular esophagomyotomy was described by Allen and Matthews 41 in 1993 but failed to achieve clinical acceptance because of its technical difficulty, perceived patient risk, and concerns over disruption of distal esophageal motility.
Collis Procedure
The Collis procedure, as a lengthening technique, has been validated clinically and is considered the standard of treatment for the short esophagus. Collis first described the operation that bears his name in 1957. 7 His goal was to create a procedure for the “frail patient” with a short esophagus who could not tolerate an esophagectomy. This lengthening procedure was performed through a thoracoabdominal incision and included mobilization of the mediastinal esophagus to the level of the aortic arch. Collis emphasized the fact that the transthoracic mobilization usually achieved sufficient intraabdominal length to allow a standard repair without tension. When additional length was needed, he created a gastric tube (the Collis gastroplasty) by dividing the stomach between two clamps. Collis did not perform a fundoplication because it was believed at that time that intraabdominal reduction of the GEJ and recreation of the acute angle of His was effective as an antireflux barrier. Unfortunately, the Collis gastroplasty alone, without a wrap, did not control reflux. 42
Collis-Belsey and Collis-Nissen
By the mid-1960s, Nissen 43 and Belsey 44 had published their methods of fundoplication for reflux control. After a few years of follow-up, it became clear that fundoplication alone was associated with unacceptably high recurrence rates in patients with a short esophagus. 4–6,45,46 Pearson et al 3 first described a transthoracic Collis-Belsey combination for patients with a shortened esophagus because “current techniques of hiatal hernia repair (Belsey/Nissen alone) may fail because the esophagogastric junction cannot be restored to an intra-abdominal position without undue tension.” They pointed out an additional advantage of this combined procedure: the fundoplication sutures are placed into the healthy tissue of the gastric tube or neoesophagus rather than into the inflamed distal esophagus. Using the combined Collis-Belsey, Pearson reported excellent results in 76% of patients followed up for 5 to 12 years. 6,47 Orringer and others, however, found problems with long-term reflux control after the Collis-Belsey and advocated a transthoracic Collis-Nissen procedure as an alternative. 48–52 The excellent results reported by Orringer’s group and others (as high as 88% symptomatic reflux control at 10 years of follow-up) have held up favorably over time, making the Collis-Nissen procedure the current gold standard for patients with a refractory short esophagus. 53–55 Nonetheless, complications related to the procedure are described in all reports and include leaks from the gastroplasty line, fistulas, and acid secretion from the ectopic gastric mucosa of the neoesophagus. 56 These complications occurred in 10% or less of open cases. 36
The Collis-Belsey and Collis-Nissen procedures have traditionally been performed through the chest. A transthoracic approach was considered necessary in patients with a short esophagus because it was difficult to perform an adequate esophageal mobilization up to the level of the aortic arch through an abdominal incision, and it was difficult to assess the amount of tension on the proximal esophagus after mobilization and repair through the abdomen. In 1986, however, Steichen 57 described an effective open transabdominal Collis-Nissen procedure using newly developed gastrointestinal stapling devices, and this has gradually become the preferred approach for lengthening procedures when the abdomen has already been opened (Fig. 4).
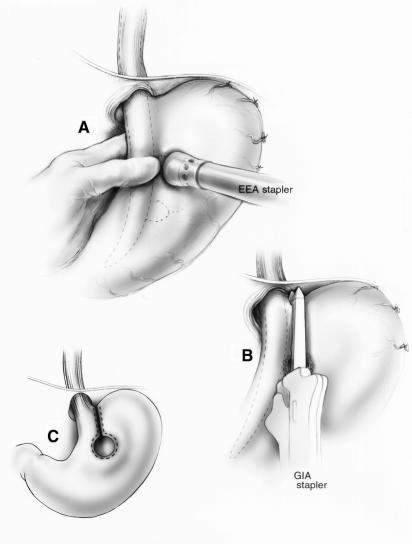
Figure 4. Steichen’s method for an open gastroplasty. (A) An EEA stapler is used to create a sealed gastric window. (B) A GIA stapler is used to create the gastroplasty. (C) Collis gastroplasty before Nissen fundoplication.
Uncut Collis Gastroplasty
Other authors have described a variation of the Collis gastroplasty that involves a vertical gastric staple line applied parallel to the esophagus. A fundoplication is then created around this uncut staple line. The uncut Collis procedure has been described as both a transthoracic and a transabdominal procedure. 58–60 The advantages advanced by those using this technique are that it minimizes leaks from the staple line of the Collis and allows improved stability of the subsequent fundoplication. This repair, however, is not truly a lengthening procedure because it primarily lengthens the intragastric component of the esophagus, and it would therefore not be suitable for a severely shortened esophagus.
Collis-Nissen in the Laparoscopic Era
Since first reported in 1991, laparoscopic techniques to perform antireflux surgery have rapidly supplanted traditional transabdominal or transthoracic approaches. This is due to the excellent exposure and visualization provided by the laparoscope and the improved patient recovery after surgery. In the early years of laparoscopic fundoplication surgery, preoperative suspicion of a short esophagus was commonly listed as a contraindication to this approach or described as an indication for conversion to an open procedure if discovered during surgery. 15 As a result of descriptions of endoscopic versions of the Collis-Nissen procedure, conversion to an open thoracic or abdominal incision is no longer required. 28,29 This development, as well as increasing comfort with laparoscopic foregut surgery, means that all requirements for a tension-free fundoplication, including a lengthening procedure when required, can be achieved while maintaining the benefits of a less invasive approach. The diagnosis of a short esophagus can be objectively confirmed, a precise mobilization of the mediastinal esophagus can be safely performed high into the mediastinum, the tension needed to reduce the GEJ can be reliably assessed, and a Collis gastroplasty can be performed when needed and a wrap fashioned around the neoesophagus.
There are still indications, of course, for an open transthoracic approach. The primary one is the surgeon’s level of experience, because an endoscopic Collis procedure demands a high level of laparoscopic skill and a detailed knowledge of esophageal anatomy and physiology. Additional indications for the open approach might be long proximal strictures, failed previous endoscopic repairs, or a hostile upper abdomen, which would make laparoscopy dangerous.
LAPAROSCOPIC COLLIS TECHNIQUES
Even if an intrinsically shortened esophagus is suspected, a standard surgical approach for laparoscopic fundoplication is used. Access to the distal esophagus is obtained by dividing the phrenoesophageal ligament, and the fundus is completely mobilized by dividing the short gastric vessels and retrogastric attachments. The distal 3 to 4 cm of esophagus is mobilized circumferentially (type I dissection) and should be the minimal dissection performed for all laparoscopic fundoplications. In 90% of cases, this dissection is sufficient to mobilize the esophagus. At this point, an assessment should be made of the intraabdominal length of esophagus; it should be 2.5 to 3 cm without tension (see Fig. 3). Care is taken to place the stomach into position for this measurement and not pull it forcibly inferiorly. Excessive traction can elongate the proximal stomach, causing it to resemble the esophagus, and this could result in a misplaced repair or a repair under tension. Intraoperative endoscopy should be used liberally to confirm the position of the GEJ when making this judgment because external assessment can be inaccurate, especially when there is fatty infiltration of the phrenoesophageal membrane or periesophageal inflammation. If 2.5 cm of intraabdominal esophagus cannot honestly be obtained after the above dissection, the patient has a short esophagus and needs a lengthening procedure.
Laparoscopic Esophageal Mobilization for the Short Esophagus
Before performing a Collis gastroplasty, extensive mediastinal mobilization of the esophagus (type II dissection) should be attempted. Using techniques developed for laparoscopic transhiatal esophagectomy, extensive dissection of the mediastinal esophagus, even to the carina, can be safely performed. 61 As originally described by Collis, this dissection will achieve sufficient intraabdominal esophageal length in most patients. After the limits of the type II dissection have been reached, another assessment of intraabdominal esophageal length is made. If 2.5 to 3 cm of intraabdominal tension-free esophagus is still not available, an endoscopic Collis gastroplasty is probably indicated. Figure 5 illustrates this pathway of patient selection for a lengthening procedure.

Figure 5. Patient selection for a lengthening procedure.
Endoscopic Collis Gastroplasty Techniques
Two endoscopic Collis gastroplasty techniques have been described. They differ from a typical transthoracic Collis gastroplasty in that they rely on laparoscopic esophageal and gastric mobilization, followed by creation of the gastroplasty and subsequent wrap, all performed intraabdominally. No descriptions have been published of a totally thoracoscopic approach to the short esophagus (i.e., a true endoscopic equivalent of the original Collis procedure).
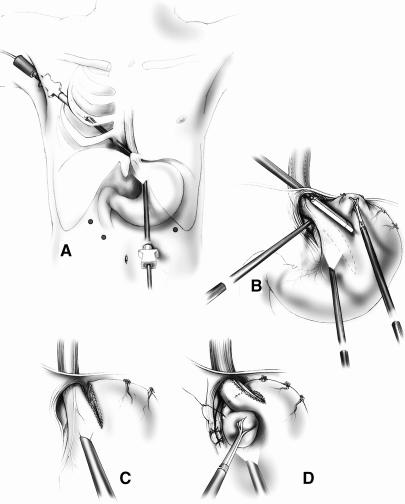
In 1996, Swanstrom et al 28 described a combined laparoscopic/thoracoscopic Collis gastroplasty. Technical aspects have been reviewed in detail elsewhere. 62 As shown in Figure 6A and B, the essential elements of this procedure involve placing a 12-mm endoscopic linear stapler into the right chest, across the right mediastinal pleura, and transhiatally into the abdomen. This allows a 3-cm stapling of the stomach parallel to the lesser curve. The resulting intraabdominal neoesophagus can subsequently be wrapped with the fundus (Fig. 6C, D).
Figure 6. (A, B) The combined thoracoscopic/laparoscopic Collis gastroplasty. (C, D) After the neoesophagus has been created, a standard fundoplication is performed around it.
A second method, using totally laparoscopic techniques, was described in 1998 by Hunter and the Emory group. 29 This technique is a laparoscopic version of the technique initially described by Steichen 57 (see Fig. 4). In this procedure, a circular stapling device is used to create a sealed “buttonhole” in the gastric fundus (Fig. 7A–C). Through this transgastric circular window, a 30-mm linear cutting stapler is then placed and fired parallel to an esophageal dilator (Fig. 7D, E). The wrap is then performed around the neoesophagus (Fig. 6C, D).
Figure 7. The double-stapled laparoscopic gastroplasty. (A–C) The sealed gastric window is created with an EEA stapler. (D, E) A linear laparoscopic GIA stapler is fired next to the bougie to create a 3-cm neoesophagus.
Each technique has advantages and disadvantages. The thoracoscopic/laparoscopic procedure involves the addition of another 12-mm port but requires only one stapler firing. It requires traversing a second body cavity (the right chest); theoretically, this could complicate anesthesia management, although it is usually done without dual-lumen intubation or special monitoring. The totally laparoscopic approach requires two different stapling devices that are more complex to position and fire, requires the creation of a generous accessory stapler insertion site, and mandates the use of larger ports to insert the second stapler. There may be specific clinical indications that would contraindicate one approach or the other (e.g., previous right chest surgery or a large left hepatic lobe), but in general both are reproducibly safe and effective, and the choice can be left to the surgeon.
ENDOSCOPIC COLLIS RESULTS
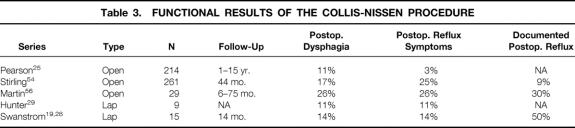
In two large, independent prospective series of patients undergoing laparoscopic fundoplications and hiatal hernia repairs, standard dissection of the distal mediastinal esophagus (type I dissection) was found to provide adequate mobilization for most patients. In one of these series, 10% of all patients had a diagnosis of a true shortened esophagus, with 7% requiring a more extensive mediastinal mobilization (type II dissection) and 3% requiring a Collis gastroplasty using the previously described thoracoscopic/laparoscopic approach. 28 In the second report, 4% of 220 patients in the series required a Collis gastroplasty using the transabdominal, double-stapling technique. 29 The mean surgical time was 257 and 294 minutes, respectively. The average length of stay for the Collis patients was 2 days in the first group and 3 in the second. Both studies reported no deaths or complications in any of the Collis patients. Functional results for both of these laparoscopic series, along with comparative open results, are shown in Table 3.
Table 3. FUNCTIONAL RESULTS OF THE COLLIS-NISSEN PROCEDURE
Theoretical concerns regarding complications of the Collis gastroplasty (open or endoscopic) have been raised. The possibility of perioperative leak at the staple line always exists, but the small (<2%) leak incidence seen with the open Collis has not yet been reported in the endoscopic literature. It has also been noted that a Collis neoesophagus typically lacks normal motility. This amotile segment may be at risk of eventual dilatation or may be a factor in postoperative dysphagia, although neither is widely described in the open or laparoscopic literature. A Collis gastroplasty also results in a minimum of 1 cm of gastric mucosa proximal to the newly reconstructed distal high-pressure zone (Fig. 8). This “ectopic” gastric mucosa is certainly nonphysiologic and has been reported in open Collis procedures to secrete acid and cause localized esophagitis. 56 To determine whether this phenomenon occurred after laparoscopic lengthening procedures, our group recently performed late objective follow-up in 15 Collis patients. 19 At 14 months after surgery, all patients underwent symptom assessment, 24-hour pH studies, manometry, and EGD with biopsy and Congo red testing of the neoesophagus. The results showed that the Collis-Nissen procedure resulted in an effective antireflux barrier as assessed by symptomatic relief, patient satisfaction, EGD results, and manometry. However, in nearly half of the patients (7/15), the neoesophagus above the wrap was found to contain parietal cells that continued to secrete acid. This was indicated by an abnormal postoperative DeMeester score and was confirmed by positive Congo red testing of the suspected mucosa. The presence of acid correlated poorly with patient complaints, which means that symptoms cannot be used as a reliable marker of acid exposure after gastroplasty.

Figure 8. A coronal section through a Collis-Nissen reconstruction. Note the presence of gastric mucosa above the new distal high-pressure zone.
Based on these data, it seems advisable to have all Collis patients closely followed up with objective testing, regardless of symptoms. If esophageal acid exposure is documented, long-term medical therapy would be indicated for this small cohort.
CONCLUSION
The clinical entity of the short esophagus was described more than 40 years ago and continues to be encountered today. Laparoscopic antireflux procedures have recently led to a renewed interest in antireflux surgery, increasing both the number of procedures being performed and the number of practitioners. This will inevitably increase the numbers of patients having a short esophagus who are referred to surgeons for treatment. Alternatively, it may also lead to better acceptance of early surgical intervention, which would reduce the overall incidence of this finding.
The Collis-Nissen procedure has an established excellent long-term success rate for this complex problem. Methods to treat the short esophagus, aside from the Collis gastroplasty, have poorer long-term results, with increased rates of complications. The most commonly practiced alternative, ignoring the short esophagus, predisposes the patient to wrap herniation, wrap disruption, or a “slipped” wrap. With the development of endoscopic Collis techniques, conversion to an open laparotomy or thoracotomy when a short esophagus is encountered is no longer necessary. The choice of the laparoscopic/thoracoscopic single-stapler technique or the laparoscopic double-stapler technique should be left to the surgeon; both procedures can be performed safely and offer all the benefits of laparoscopic surgery. Even though a lengthening procedure is the best choice for a patient with a true shortened esophagus, it is a nonphysiologic treatment for a complex problem and requires long-term follow-up.
Acknowledgment
The authors thank Corinne Sandone for the illustrations.
Footnotes
Correspondence: Karen D. Horvath, MD, Dept. of Surgery, University of Washington, 1959 NE Pacific St., Seattle, WA 98195.
Reprints will not be available.
E-mail: khorvath@u.washington.edu
Accepted for publication July 12, 2000.
References
- 1.DeMeester TR, Hubert S. Surgical treatment of gastroesophageal reflux disease. In: Castell DC, eds. The Esophagus. Boston: Little Brown; 1992: 579–626.
- 2.Orringer MB. Reflux stricture and short esophagus. In: Zuidema GD, Orringer MB, eds. Shackleford’s Surgery of the Alimentary Tract, 3d ed. Philadelphia: W.B. Saunders, 1986: 222.
- 3.Pearson FG, Langer B, Henderson RD. Gastroplasty and Belsey hiatus hernia repair: an operation for the management of peptic stricture with acquired short esophagus. J Thorac Cardiovasc Surg 1971; 61: 50–63. [PubMed] [Google Scholar]
- 4.Orringer MB, Skinner DB, Belsey RH. Long-term results of the Mark IV operation for hiatal hernia and analyses of recurrences and their treatment. J Thorac Cardiovasc Surg 1972; 63: 25. [PubMed] [Google Scholar]
- 5.Krupp S, Rossetti M. Surgical treatment of hiatal hernias by fundoplication and gastropexy (Nissen repair). Ann Surg 1966; 182: 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson FG. Surgical management of acquired short esophagus with dilatable peptic stricture. World Surg 1977; I: 463. [DOI] [PubMed] [Google Scholar]
- 7.Collis JL. An operation for hiatus hernia with short esophagus. Thorax 1957; 12: 181–188. [PMC free article] [PubMed] [Google Scholar]
- 8.Watson DI, Jamieson GG, Game PA, Williams RS, Devitt PG. Laparoscopic reoperation following failed antireflux surgery. Br J Surg 1999; 86: 98–101. [DOI] [PubMed] [Google Scholar]
- 9.Hinder RA, Filipi CJ, Wescher G, et al. Laparoscopic Nissen fundoplication is an effective treatment for gastroesophageal reflux disease. Ann Surg 1994; 220: 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cadiere GB, Houben JJ, et al. Laparoscopic Nissen fundoplication: technique and preliminary results. Br J Surg 1994; 81: 400–403. [DOI] [PubMed] [Google Scholar]
- 11.Peters JH, Heimbucher J, Kauer WKH, et al. Clinical and physiologic comparison of laparoscopic and open Nissen fundoplication. J Am Coll Surg 1995; 180: 385–393. [PubMed] [Google Scholar]
- 12.Hunter JG, Swanstrom LL, Waring JP. Dysphagia after laparoscopic antireflux surgery: the impact of operative technique. Ann Surg 1996; 224: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evangelist FA, Taylor FFH, Alford JD. The modified Collis-Nissen operation for control of gastroesophageal reflux. Ann Thorac Surg 1978; 26: 107–111. [DOI] [PubMed] [Google Scholar]
- 14.Patti MG, Arcerito M, Feo CV, et al. An analysis of operations for gastroesophageal reflux disease. Arch Surg 1998; 133: 600–607. [DOI] [PubMed] [Google Scholar]
- 15.Peters JH, DeMeester TR. The lessons of failed antireflux repairs. In: Peters JH, DeMeester TR, eds. Minimally Invasive Therapy of the Foregut. St. Louis: Quality Medical Publishing; 1994: 190.
- 16.Siewert JR, Isolauri J, Feussner H. Reoperation following failed fundoplication. World J Surg 1989; 13: 791–797. [DOI] [PubMed] [Google Scholar]
- 17.Ellis FH, Gibb SP, Heatley GJ. Reoperation after failed antireflux surgery: review of 101 cases. Eur J Cardiothorac Surg 1996; 10: 225–232. [DOI] [PubMed] [Google Scholar]
- 18.DePaula AL, Hashiba K, Bajutto M, Machado CA. Laparoscopic reoperations after failed and complicated antireflux operations. Surg Endosc 1995; 9: 681–686. [DOI] [PubMed] [Google Scholar]
- 19.Jobe BA, Horvath KD, Swanstrom LL. Postoperative function following laparoscopic Collis gastroplasty for the shortened esophagus. Arch Surg 1998; 133: 867–874. [DOI] [PubMed] [Google Scholar]
- 20.Stirling MC, Orringer MB. Surgical treatment after the failed antireflux operation. J Thorac Cardiovasc Surg 1986; 92: 667–672. [PubMed] [Google Scholar]
- 21.Bremner RM, Crookes PF, Costantini M, DeMeester TR, Peters JH. The relationship of esophageal length to hiatal hernia in gastroesophageal reflux disease. Gastroenterology 1992; 102: A45. [Google Scholar]
- 22.Coster DD, Bower W, Wilson VT, Brebrick RT, Richardson GL. Laparoscopic partial fundoplication vs. laparoscopic Nissen-Rossetti fundoplication: short-term results of 231 cases. Surg Endosc 1997; 11: 625–631. [DOI] [PubMed] [Google Scholar]
- 23.Hill LD, Gelfand M, Bauermeister D. Simplified management of reflux esophagitis with stricture. Ann Surg 1970; 172: 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam CR, Gahagan TH. The myth of the short esophagus. In: Nyhus LM, Harkins HN, eds. Hernia. Philadelphia: JB Lippincott; 1964: 450.
- 25.Pearson FG, Todd TR. Gastroplasty and fundoplication for complex reflux problems: long-term results. Ann Surg 1987; 206: 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kauer WKH, Peters JH, DeMeester TR, Heimbucher J, Ireland AP, Bremner CG. A tailored approach to antireflux surgery. J Thorac Cardiovasc Surg 1995; 110: 141–147. [DOI] [PubMed] [Google Scholar]
- 27.Polk HC. Fundoplication for reflux esophagitis: misadventures with the operation of choice. Ann Surg 1976; 183: 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swanstrom LL, Marcus DR, Galloway GQ. Laparoscopic Collis gastroplasty is the treatment of choice for the shortened esophagus. Am J Surg 1996; 171: 477–481. [DOI] [PubMed] [Google Scholar]
- 29.Johnson AB, Oddsdottir M, Hunter JG. Laparoscopic Collis gastroplasty and Nissen fundoplication: a new technique for the management of esophageal foreshortening. Surg Endosc 1998; 12: 1055–1060. [DOI] [PubMed] [Google Scholar]
- 30.Pearson FG. Peptic esophagitis, stricture and short esophagus. In: Esophageal Surgery. New York: Churchill Livingstone; 1995.
- 31.Jobe BA, Horvath KD, Bennetts W, Swanstrom LL. An objective measure of the shortened esophagus prior to antireflux surgery (unpublished data).
- 32.Moghissi I. Intrathoracic fundoplication for reflux stricture associated with short esophagus. Thorax 1983; 38: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pennell T. Supradiaphragmatic correction of esophageal reflux strictures. Ann Surg 1981; 193: 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safaie-Shirazi S, Sike WL, Anuras S, et al. Nissen fundoplication without crural repair. Arch Surg 1974; 108: 424. [DOI] [PubMed] [Google Scholar]
- 35.Burnett HF, Read RC, Morris WB, et al. Management of complications of fundoplication and Barrett’s esophagus. Surgery 1977; 82: 521. [PubMed] [Google Scholar]
- 36.Mansour K, Burton H, Miller J, et al. Complications of intrathoracic Nissen fundoplication. Ann Thorac Surg 1981; 32: 173–178. [DOI] [PubMed] [Google Scholar]
- 37.Rossman F, Brantigan CO, Sawyer RB. Obstructive complications of the Nissen fundoplication. Am J Surg 1979; 138: 360. [DOI] [PubMed] [Google Scholar]
- 38.Richardson J, Larson G, Polk H. Intrathoracic fundoplication for shortened esophagus. Treacherous solution to a challenging problem. Am J Surg 1982:; 43:29–35. [DOI] [PubMed]
- 39.Balison J, McGregor A, Woodward E. Postoperative diaphragmatic herniation following transthoracic fundoplication. Arch Surg 1973; 106: 164–166. [DOI] [PubMed] [Google Scholar]
- 40.Ritter MP, Peters JH, De Meester TR, et al. Treatment of advanced gastroesophageal reflux disease with Collis gastroplasty and Belsey partial fundoplication. Arch Surg 1998; 133: 523–528. [DOI] [PubMed] [Google Scholar]
- 41.Allen SM, Matthews HR. Circular myotomy and Belsey repair for acquired shortening of the oesophagus. Eur J Cardiothorac Surg 1993; 7: 645–647. [DOI] [PubMed] [Google Scholar]
- 42.Adler RH. Collis gastroplasty: origin and evolution. Ann Thorac Surg 1990; 50: 839–842. [DOI] [PubMed] [Google Scholar]
- 43.Nissen R. Gastropexy and “fundoplication” in surgical treatment of hiatal hernia. Am J Dig Dis 1961; 6: 954–961. [DOI] [PubMed] [Google Scholar]
- 44.Skinner D, Belsey RH. Surgical management of esophageal reflux and hiatus hernia. J Thorac Cardiovasc Surg 1967; 53: 33–54. [PubMed] [Google Scholar]
- 45.Donnelly RJ, Deverall B, Watson DA. Hiatal hernia with and without esophageal stricture: experience with Belsey Mark IV repair. Ann Thorac Surg 1973; 16: 301. [DOI] [PubMed] [Google Scholar]
- 46.Hollenbeck JI, Woodward ER. Treatment of peptic esophageal stricture with combined fundic patch-fundoplication. Ann Surg 1975; 182: 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pearson FG, Henderson, RD. Long-term follow-up of peptic strictures managed by dilatation, modified Collis gastroplasty and Belsey hiatus hernia repair. Surgery 1976; 80: 396. [PubMed] [Google Scholar]
- 48.Orringer MB, Sloan H. Complications and failings of the combined Collis-Belsey operation. J Thorac Cardiovasc Surg 1977; 74: 726. [PubMed] [Google Scholar]
- 49.Henderson RD. Reflux control following gastroplasty. Ann Thorac Surg 1977; 24: 206. [DOI] [PubMed] [Google Scholar]
- 50.Henderson RD, Marryatt GV. Total fundoplication gastroplasty (Nissen gastroplasty): five-year review. Ann Thorac Surg 1985; 39: 74. [DOI] [PubMed] [Google Scholar]
- 51.Orringer MB, Orringer JS. The combined Collis-Nissen operation: early assessment of reflux control. Ann Thorac Surg 1982; 33: 534. [DOI] [PubMed] [Google Scholar]
- 52.Orringer MB, Sloan H. Combined Collis-Nissen reconstruction of the esophagogastric junction. Ann Thorac Surg 1978; 25: 16. [DOI] [PubMed] [Google Scholar]
- 53.Orringer MB, Sloan H. Combined Collis-Nissen reconstruction of the esophagogastric junction. Ann Thorac Surg 1978; 25: 16–21. [DOI] [PubMed] [Google Scholar]
- 54.Stirling MC, Orringer MB. Continued assessment of the combined Collis-Nissen operation. Ann Thorac Surg 1989; 47: 224–230. [DOI] [PubMed] [Google Scholar]
- 55.Stirling MC, Orringer MB. The combined Collis-Nissen operation for esophageal reflux strictures. Ann Thorac Surg 1988; 45: 148. [DOI] [PubMed] [Google Scholar]
- 56.Martin CJ, Cox MR, Cade RJ. Collis-Nissen gastroplasty fundoplication for complicated gastro-oesophageal reflux disease. Aust NZ J Surg 1992; 62: 126–129. [DOI] [PubMed] [Google Scholar]
- 57.Steichen FM. Abdominal approach to the Collis gastroplasty and Nissen fundoplication. Surg Gynecol Obstet 1986; 162: 372–374. [PubMed] [Google Scholar]
- 58.Demos NJ. Stapled, uncut gastroplasty for hiatal hernia: 12-year follow-up. Ann Thorac Surg 1984; 38: 393–399. [DOI] [PubMed] [Google Scholar]
- 59.Trastek VF. Uncut Collis-Nissen gastroplasty. Chest Surg Clin North Am 1995; 5: 423–435. [PubMed] [Google Scholar]
- 60.Cameron BH, Cochran WJ, McGill CW. The uncut Collis-Nissen fundoplication: results for 79 consecutively treated high-risk children. J Pediatr Surg 1997; 32: 887–891. [DOI] [PubMed] [Google Scholar]
- 61.Swanstrom LL, Hansen P. Laparoscopic total esophagectomy. Arch Surg 1997; 132: 943–949. [DOI] [PubMed] [Google Scholar]
- 62.Horvath KH, Swanstrom LL. Endoscopic esophageal lengthening procedures for the shortened esophagus: the combined laparoscopic/thoracoscopic Collis gastroplasty. In: Zucker CA, ed. Surgical Laparoscopy, 3d ed. Baltimore: Williams & Wilkins (in press).