Abstract
Objective
To explore the hypothesis that oxandrolone may reverse muscle catabolism in cachectic, critically ill pediatric burn patients.
Summary Background Data
Severe burn causes exaggerated muscle protein catabolism, contributing to weakness and delayed healing. Oxandrolone is an anabolic steroid that has been used in cachectic hepatitis and AIDS patients.
Methods
Fourteen severely burned children were enrolled during a 5-month period in a prospective cohort analytic study. There was a prolonged delay in the arrival of these patients to the burn unit for definitive care. This neglect of skin grafting and nutritional support resulted in critically ill children with significant malnutrition. On arrival, all patients underwent excision and skin grafting and received similar clinical care. Subjects were studied 5 to 7 days after admission, and again after 1 week of oxandrolone treatment at 0.1 mg/kg by mouth twice daily or no pharmacologic treatment. Muscle protein kinetics were derived from femoral arterial and venous blood samples and vastus lateralis muscle biopsies during a stable isotope infusion.
Results
Control and oxandrolone subjects were similar in age, weight, and percentage of body surface area burned. Muscle protein net balance decreased in controls and improved in the oxandrolone group. The improvement in the oxandrolone group was associated with increased protein synthesis efficiency. Muscle protein breakdown was unchanged.
Conclusions
In burn victims, oxandrolone improves muscle protein metabolism through enhanced protein synthesis efficiency. These findings suggest the efficacy of oxandrolone in impeding muscle protein catabolism in cachectic, critically injured children.
Severe burns induce marked physiologic derangements in addition to skin injury. These include hypermetabolism with erosion of lean body mass, generalized weakness, altered immune function with increased infectious complications, peripheral insulin resistance, and poor wound healing. 1–6 Many therapeutic strategies have been used to attenuate the hypermetabolic response, and the few notable successes have improved clinical outcome in burn care. Heating the patient environment to 88°F (30°C) to correspond with the patient’s elevated hypothalamic temperature set point has been shown to decrease the metabolic rate. 7 Early excision of burned and dead tissue to stop further inflammatory stimulation has decreased length of stay, incidence of sepsis, and blood loss associated with skin grafting. 8,9 However, even with these improvements, survivors of severe burn are hypermetabolic for a prolonged period (up to 8 months after burn), 10 with profound muscle wasting, decreased bone mineral density, and (in children) retarded linear growth as negative clinical consequences. 11–13
To avert hypermetabolism, pharmacologic agents have been used to stimulate growth and increase strength after burn. Recombinant human growth hormone has shown efficacy in improving muscle protein kinetics 14–17 and wound healing, 18–20 and also in lowering the death rate in severely burned patients. 21 However, it requires a daily subcutaneous injection and can cause hyperglycemia. 14,18 Recently, questions have been raised about its safety in adult patients in the intensive care unit setting. 22,23 Insulin has beneficial results on protein metabolism. At low doses, its action is directed at attenuating protein breakdown, but at higher doses it primarily affects protein synthesis. 24–26 The potential for precipitating hypoglycemia must be carefully weighed before beginning exogenous insulin treatment in the face of clinical euglycemia. Testosterone can increase protein synthesis, 27 but its use entails risks of virilism and hepatotoxicity.
Oxandrolone, an oral synthetic testosterone analog, has been used in acute and rehabilitating adult burn patients with promising results in terms of weight gain and urinary nitrogen balance. 28,29 It also has been used successfully in children with Turner syndrome and other constitutional delays in growth, in cachectic alcoholic hepatitis patients, and in patients with AIDS wasting myopathy. 30–32 Compared with testosterone, it has minimal virilizing activity and little hepatotoxicity. 33,34
The purpose of our study was to determine whether oxandrolone would affect burn-induced catabolism—in other words, would it improve the net balance of muscle protein synthesis and breakdown—and if so, by what mechanism. As an international tertiary referral center for children with severe burns, we receive many patients who are weeks removed from their injury. These patients arrive without definitive surgical treatment or adequate nutritional support. Burn wound sepsis or chronic eschar colonization is frequent. These children with large (20–95% total body surface area [TBSA]) burns, chronic infection, and nutritional depletion are critically ill and extremely catabolic on arrival to our burn unit. We chose to study this subpopulation of burn victims because they shared a similar therapeutic indication (depleted nutritional status) with patients who had received oxandrolone in previous studies. 31,32
METHODS
Patients
This study was performed under a protocol approved by the University of Texas Medical Branch Institutional Review Board. Informed written consent was obtained from each patient’s guardian before study enrollment. Inclusion criteria were as follows: younger than 18 years of age, burns of greater than 20% TBSA, and nutritional depletion based on the clinical findings of the physical examination and below-normal values of serum nutritional indices (albumin, prealbumin, retinol-binding protein, transferrin).
All subjects were admitted to the Shriners Burns Hospital for Children in Galveston, Texas, at least one week after injury. Within 48 hours of admission, each patient underwent total burn wound excision and grafting with autograft skin and allograft. Patients were returned to the operating room when autograft donor sites healed and became available for reharvest (usually 6–10 days). Sequential staged surgical procedures for repeat excision and grafting were undertaken until the wounds were healed.
Each patient received enteral nutrition by nasoduodenal intubation with Vivonex TEN (Sandoz Nutritional Corp., Minneapolis, MN). The composition of Vivonex is 82% carbohydrate, 15% protein, and 3% fat. Daily caloric intake was given at a rate calculated to deliver 1,500 kcal/m2 TBSA burned + 1,500 kcal/m2 TBSA. This feeding regimen was started at admission and continued at a constant rate until the wounds were healed. Caloric intake remained constant throughout the study periods.
Patients were at bed rest after excision and grafting procedures for 5 days. After this, patients ambulated daily until the next excision and grafting procedure. Patients were treated in an identical fashion in terms of mobilization and rehabilitation in both study periods.
Study Design
From February 1999 through July 1999, 14 patients were enrolled in a prospective cohort analytic series. The first seven received oxandrolone treatment, and the next seven served as time controls.
After the first surgical procedure, all patients were studied on postoperative day 5 to determine baseline protein metabolism (Fig. 1). Net phenylalanine balance across the leg and the fractional synthetic rate (FSR) of skeletal muscle protein were measured as main outcome variables. When the donor sites healed at 6 to 10 days, patients were returned to the operating room for another excision and grafting procedure. After the second surgical procedure, patients in the drug treatment group received oxandrolone 0.1 mg/kg twice daily for 5 days and control patients received no anabolic agents for 5 days. A second series of protein kinetic studies was performed on postoperative day 5 after the second surgical procedure to determine any differences with oxandrolone treatment or time between the drug and control patients.
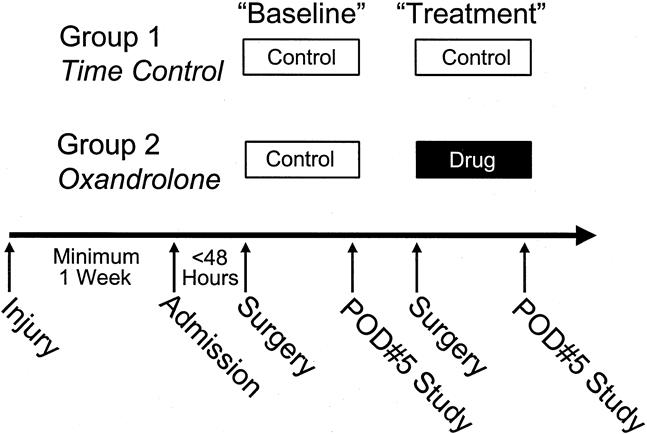
Figure 1. Study design. This study comprised nutritionally depleted children burned over 20% total body surface area who were at least 1 week removed from injury. Burn wounds were totally excised and grafted within 48 hours of admission. After 5 days, a baseline muscle protein kinetic study was done. After the second serial grafting procedure, patients in the control and drug groups underwent a 5-day treatment period and then were studied again.
Oxandrolone was purchased from BTG Pharmaceuticals (Iselin, NJ). It was administered by mouth to the patients in the drug treatment group if tolerated. Otherwise, the oxandrolone tablet was crushed and suspended in 2 mL ethanol. This suspension was then injected into the nasoduodenal tube, followed by a 20-mL flush of sterile water.
Stable Isotope Infusion Protocol
On postoperative day 5, all patients underwent a 5-hour protein kinetic study (Fig. 2). Baseline blood samples were obtained from an indwelling femoral arterial or central venous catheter for background amino acid enrichment and systemic indocyanine green (ICG) concentrations. A primed-constant infusion of L-[ring-2H5]-phenylalanine (Cambridge Isotopes, Andover, MA) was given through the central venous catheter for 5 hours, using a priming dose of 2 μmol/kg followed by 0.08 μmol/kg/min. Because phenylalanine is not synthesized or degraded in the peripheral tissues (it is metabolized only in the liver), measurement across the leg reflects the net balance of protein synthesis and breakdown. Vastus lateralis muscle biopsies were taken from the study leg after 2 and 5 hours while the subject was under intravenous conscious sedation with midazolam (Versed; Roche Pharmaceuticals, Nutley, NJ) and propofol (AstraZeneca Pharmaceuticals, Wilmington, DE) and local anesthesia with 1% lidocaine without epinephrine (Abbot Laboratories, Chicago, IL). The muscle biopsies were performed using a Bergstrom needle (Depuy, Chicago, IL) attached to a suction device. Samples were immediately washed with normal saline to remove any blood, blotted dry, and then snap-frozen in liquid nitrogen for storage at -70°C. Between hours 3 and 4, leg blood flow was determined by ICG infusion (infusion rate 0.5 mg/min) into the femoral artery. Blood samples from the femoral and subclavian veins were taken simultaneously every 15 minutes for this determination. Between hours 4 and 5, blood samples were obtained from the femoral artery and vein to determine arteriovenous phenylalanine concentration differences across the leg. After the last muscle biopsy, the stable isotope infusion was stopped. Catheters were left in place for use at the next excision and grafting procedure.
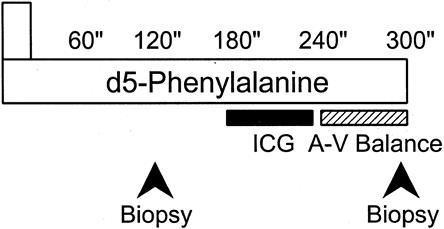
Figure 2. Five-hour isotope infusion protocol. Beginning between 5 and 7 am, subjects in the continuously fed state were infused with a prime-constant d5-phenylalanine isotope. Indocyanine green dye concentration was measured between hours 3 and 4 to determine leg blood flow. Femoral arteriovenous sampling during the fifth hour measured cross-leg phenylalanine balance. Muscle biopsies were performed at 2 and 5 hours while subjects were under intravenous conscious sedation.
Analysis of Samples
Blood
The blood concentration of unlabeled phenylalanine and the enrichment of its isotopic counterpart were simultaneously determined by gas chromatography–mass spectrometry using the internal standard approach and the nitrogen-acetyl-n-propyl esters, as previously described. 35 Briefly, 2 mL whole blood was immediately mixed with 200 μL internal standard containing 29.7 μmol/L [13C6] phenylalanine. The samples were deproteinized with sulfosalicylic acid and centrifuged, and the supernatant was processed to form nitrogen-acetyl-n-propyl esters. The isotopic enrichment of free amino acids in blood was determined on an HP Model 5989 (Hewlett-Packard Co., Palo Alto, CA) by chemical ionization and selected ion monitoring at mass-to-charge ratios of 250.1, 255.1, and 256.1. 36 Finally, ICG concentrations were determined spectrophotometrically at λ=805 nm on a Spectronic 1001 (Bausch & Lomb, Rochester, NY).
Muscle
Tissue biopsies of the vastus lateralis were immediately blotted and frozen in liquid nitrogen. Samples were then stored at -70°C until processed. Each tissue sample was weighed and protein was precipitated with a 5% perchloric acid solution. In this process, an internal standard containing 5.9 μmol/L [13C6] phenylalanine was added and thoroughly mixed. The tissues were then homogenized and centrifuged, and the supernatant fluid was processed as described for the plasma samples above. The pellet of protein precipitate was washed twice with 10 mL 2% perchloric acid followed by a series of washes (twice with absolute ethanol, once with ether) to eliminate free amino acids and lipids. After these tissue samples were dried in an oven at 50°C overnight, the dry weight of tissues was measured and total water content was obtained from the difference between weight of the wet and the dried sample. The protein precipitate was hydrolyzed at 110°C for 36 hours in constant boiling hydrochloric acid. N-heptafluorobutyryl-n-propylester derivatives were formed and dissolved in a final volume of 100 μL ethyl acetate. One-microliter aliquots were assayed in triplicate on a VG 12-250 gas chromatography/mass spectrometry system (Fisons, East Grinstead, England) using 15 m × 0.32 mm DB5-MS capillary column. Enrichments were determined by comparing the measured M + 5/M + 3 isotopomer ratio of samples to a set of 13C6-phenylalanine isotope dilution calibration standards.
Plasma Amino Acid Concentrations
Arterial and venous amino acid concentrations were determined in heparinized plasma at 4 and 5 hours of the isotope infusion study. Amino acid concentrations were determined by high-performance liquid chromatography. 37
Clinical Chemistry
On the morning of both the baseline and treatment studies, 13 mL whole blood was drawn from the patient and sent for a liver function panel, a nutrition panel, and coagulation times. The liver panel and nutrition panels were sent in nonheparinized tubes and centrifuged at 2,500 rpm for 10 minutes. The serum fraction was analyzed on a Kodak Ektachem 400 (Eastman Kodak, Billerica, MA) for the liver panel and on a Behring Nepholometer 100 (Roche Diagnostics, Indianapolis, IN) for the prealbumin, transferrin, and retinol-binding protein levels. The coagulation panel was sent in a citrated blood tube and centrifuged at 4,000 rpm for 10 minutes to separate the plasma fraction. This was analyzed on an ACL #100 automated coagulation system (Beckman Coulter, Fullerton, CA) to determine prothrombin time.
Energy Expenditure
Energy expenditure was measured by indirect calorimetry. Between 2 and 5 am on the day of study in both the baseline and treatment periods, CO2 production, O2 uptake, respiratory quotient, and resting energy expenditure were measured with a standard metabolic cart (Sensormedics Model 2900, Yorba Linda, CA). Values were accepted when they were at a steady state for 5 minutes and their standard deviations were less than 10% of their respective measures.
Calculations
Leg Blood Flow
Leg blood flow was determined from the following modification of Fick’s equation:
where CF is the femoral venous concentration of ICG and CC is the central venous concentration of ICG. The infusion rate was known (0.5 mg/min), so the equation was solved for leg blood flow (BF). For protein kinetic calculations, BF was normalized for each patient by dividing by the patient’s leg volume. In the rest of this article, units of BF will be mL/min/100-mL leg.
Kinetic Model
Leg amino acid kinetics were calculated according to a three-compartment model, as previously described. 38 The three-compartment model parameters were calculated as follows:
where CA and CV are the blood free amino acid concentrations of the femoral artery and vein, respectively; EA, EV, and EM are amino acid enrichments (tracer/tracee ratio) in the femoral artery, vein, and the free intracellular muscle pool, respectively; and BF is leg blood flow in mL/min/100-mL leg.
Fractional Synthetic Rate of Muscle Protein
The skeletal muscle FSR was calculated from the determination of the rate of d5-labeled phenylalanine incorporation into the protein and the enrichment of the intracellular pool as the precursor by the following equation 39:
where EB1 and EB2 are the fractions of the d5-tagged phenylalanine that have been bound into muscle protein at time points 1 (2 hours) and 2 (5 hours) respectively, and t is the exact time in minutes between the sampling points.
Statistical Analysis
Data are presented as means ± standard error. Paired t tests were used to compare baseline and treatment data within each group. Comparisons between the oxandrolone and time control treatment periods were made by unpaired t tests. P < .05 was considered statistically significant.
RESULTS
Patient characteristics are listed in Table 1. The seven control and seven oxandrolone patients were similar in age, sex, weight, percentage TBSA burned, and percentage of third-degree burns. Both groups had delayed presentation to our burn unit of approximately 4 to 6 weeks after injury. All subjects arrived with open burn wounds that had not received definitive surgical treatment (excision of burn and split-thickness skin grafting). The baseline and treatment stable isotope studies were performed at the same times relative to injury in both groups.
Table 1. PATIENT CHARACTERISTICS
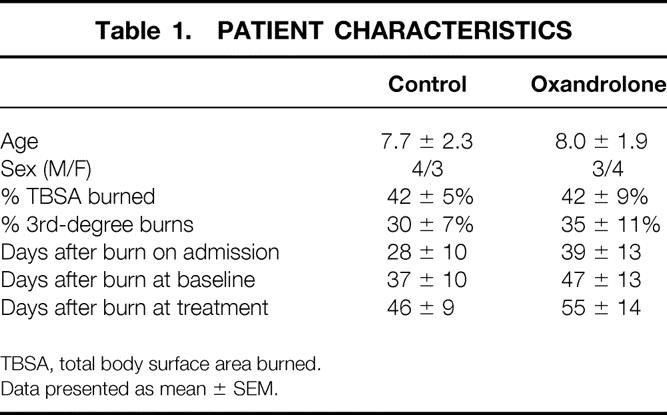
TBSA, total body surface area burned.
Data presented as mean ± SEM.
Nutritional Evaluation
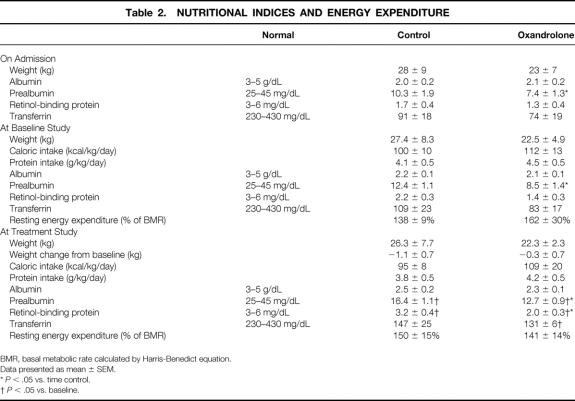
Nutritional data and energy expenditure are listed in Table 2. These patients all appeared malnourished, with low body weight in relation to height, temporalis muscle wasting, and exaggerated skeletal prominences. All of them arrived with low albumin, prealbumin, and retinol-binding protein levels. The prealbumin levels in the oxandrolone group were lower than those in the control group, but both groups were profoundly low. After 1 week of continuous enteral feeding, the caloric and protein intakes were the same in both groups, and the levels of the nutritional indices increased. These values were still far below normal, however, and the oxandrolone group was still lower than the control group.
Table 2. NUTRITIONAL INDICES AND ENERGY EXPENDITURE
BMR, basal metabolic rate calculated by Harris-Benedict equation.
Data presented as mean ± SEM.
* P < .05 vs. time control.
† P < .05 vs. baseline.
During the second week (the treatment time period), dietary intake was again isocaloric and isonitrogenous. At the time of the treatment studies, all nutritional indices had again improved, but they were still below normal. Prealbumin and retinol-binding protein levels were lower in the oxandrolone group. The oxandrolone and control patients showed the same degree of improvement over time, however.
Energy Expenditure
All subjects were hypermetabolic as measured by indirect calorimetry. In each patient, the resting energy expenditure was above the predicted basal metabolic rate from the Harris-Benedict equation. There were no differences between groups or at different time periods.
Protein Kinetics
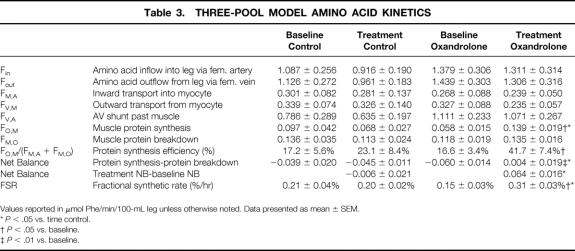
Model-derived values are listed in Table 3. Oxandrolone administration significantly increased muscle protein net balance from baseline and from time-matched control patients (P < .01 vs. baseline and P < .05 vs. time control, Fig. 3). This improvement was due to an increased rate of protein synthesis (P < .05). Protein breakdown was unchanged from baseline levels. The inward transport of amino acids from blood into muscle cells was unchanged by oxandrolone administration. However, the protein synthesis efficiency (the fraction of the available intracellular pool of amino acids directed toward synthesis) was increased compared with baseline and with time control (P < .05). The FSR, as calculated by direct incorporation of labeled phenylalanine into skeletal muscle protein, independently confirmed the increase in protein synthesis after oxandrolone administration compared with baseline and time control (P < .05).
Table 3. THREE-POOL MODEL AMINO ACID KINETICS
Values reported in μmol Phe/min/100-mL leg unless otherwise noted. Data presented as mean ± SEM.
* P < .05 vs. time control.
† P < .05 vs. baseline.
‡ P < .01 vs. baseline.
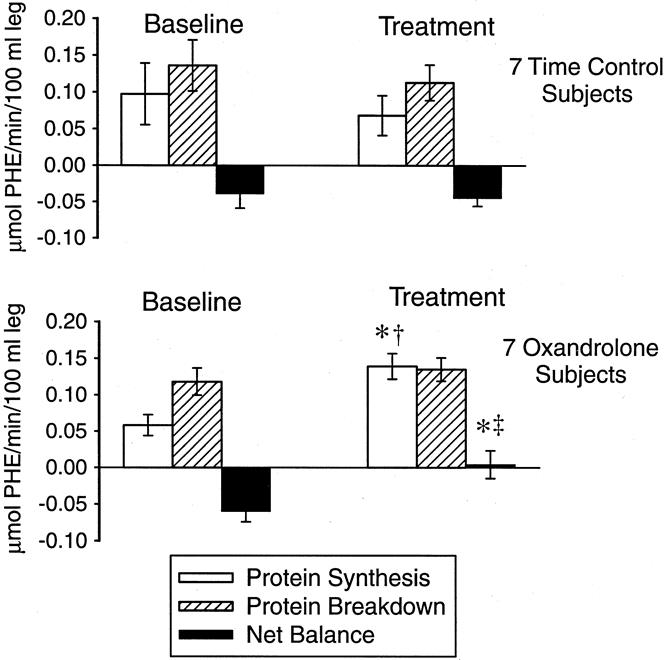
Figure 3. Model calculations of protein synthesis, protein breakdown, and net balance for seven oxandrolone and seven control patients. †P < .05 vs. baseline; ‡P < .01 vs. baseline; * P < .05 vs. time control (treatment period).
Clinical Factors
No hirsutism, acne, or behavior changes were noted in boys or girls receiving oxandrolone. Results of liver function tests are listed in Table 4. There were no differences between the groups. No patient developed clinically significant hepatic dysfunction.
Table 4. LIVER FUNCTION TEST RESULTS
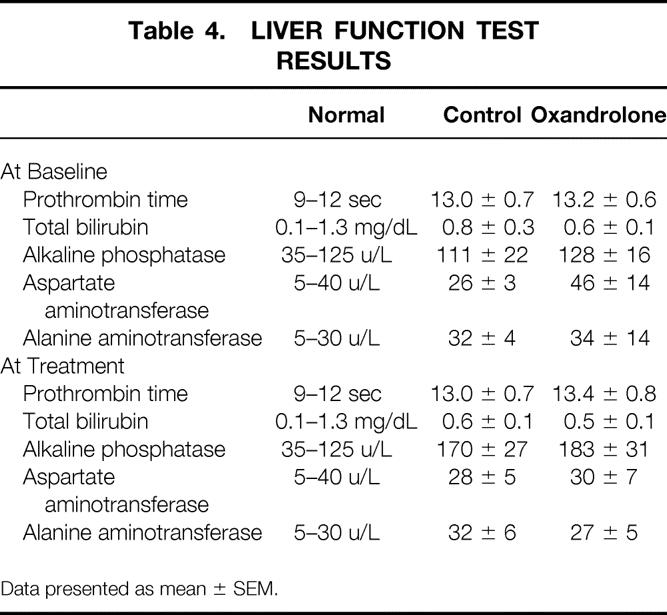
Data presented as mean ± SEM.
There were no deaths. One patient in each group appeared septic for a portion of the hospital course. The septic oxandrolone patient arrived with frank burn wound sepsis (diagnosed by hemodynamic and other clinical parameters consistent with sepsis syndrome, Staphylococcus bacteremia, and wound tissue counts of >106Staphylococcus aureus). She was treated with immediate, total excision of her 95% TBSA burn wound and systemic antibiotics. Her clinical sepsis resolved 3 weeks later while receiving oxandrolone. The septic patient in the control group arrived critically ill, with hyperthermia, hypoxemia, a hyperdynamic circulation, bilateral tube thoracostomies, and clinical and x-ray indications of a left-sided pneumonia. A panresistant Pseudomonas species was cultured from his blood and sputum. After immediate total excision of his burn wound and 2 weeks of systemic antibiotic therapy, his clinical sepsis resolved.
DISCUSSION
In burn-induced catabolism, protein breakdown is accelerated and protein synthesis is decreased—thus, breakdown outweighs synthesis and the net balance is negative. For the first time in burn patients, we applied stable isotope methodology to show that oxandrolone alters this balance by increasing the synthetic component of this two-sided equation. Of 14 severely burned, nutritionally depleted children who underwent delayed definitive surgical treatment, 7 received oxandrolone (0.1 mg/kg by mouth twice daily) and exhibited increased efficiency of protein synthesis within muscle cells.
We enrolled 7 burned boys and 7 burned girls into this study. On admission, these children all appeared emaciated and had laboratory values consistent with nutritional depletion. All had below-normal levels of albumin, prealbumin, retinol-binding protein, and transferrin. Despite our best care with aggressive nutritional support and early definitive surgical treatment, after 1 week all patients still had below-normal values of all measured nutritional indices. Even after a second week of hospital care with continuous enteral feedings, all subjects were still well below normal in their nutritional indices. We view this as a testament to the difficulty of nutritional and general clinical support of these children. It is certain that the referring hospitals and physicians did not intentionally underfeed or starve any of these patients. Rather, we believe that nutritional support of severely burned children is a difficult task, with no dramatic effects on hypermetabolism.
The effects of enteral nutrition on the hypermetabolic state associated with severe burn are controversial. In the early 1980s, the results of several well-controlled small animal studies suggested that prompt (<1 hour after burn) institution of enteral feeding attenuated the hypermetabolic response and its attendant clinical complications (protein catabolism, erosion of body mass, disturbed gut mucosal integrity). 40–42 The conclusion that early, aggressive enteral nutritional support decreased hypermetabolism and improved clinical outcome was readily extrapolated by burn and trauma surgeons to their injured patients. Today, the impression of these studies persists in the therapeutic philosophies of many surgeons. Unfortunately, no human trial has ever documented such an alteration in the metabolic response to burn with early, aggressive enteral feeding. The nutritionally depleted, burned children described here did not experience a decrease in their postburn resting energy expenditure despite aggressive enteral nutritional support. Our feeding regimen was successful in delivering a high-calorie and high-protein intake and in improving overall nutritional status, as shown by the increased nutritional indices.
All of these patients arrived with intact burn eschar. In a recent examination of our patient population and clinical burn practice, we found that a delay in definitive surgical therapy (complete burn wound excision and split-thickness skin grafting) correlated with a greater degree of catabolism by linear regression analysis (unpublished data). Relative to all of our previously reported series of burn patients, the patients in this study were more catabolic at baseline.
To improve the protein kinetic state in the emaciated burned children described here, we chose to test the effects of the anabolic steroid oxandrolone. In a recent prospective randomized trial that involved recuperating, severely burned adults, Demling and DeSanti 28 reported that this oral anabolic agent improved weight gain and also a nonquantified measure of muscle function in seven rehabilitating severely burned adults compared with six similar rehabilitating control patients. Although that study was prospective and randomized, the outcome measures were not definitive. Net weight change is affected by many clinical factors (importantly, hydration and fluid shifts) and thus is generally regarded as a poor indicator of true anabolism or catabolism. The reported improvement in muscle function in that study was based on the subjective, nonquantified evaluation by a nonmasked physical therapist. Another trial by the same author was published last year and showed a decrease in weight loss and urinary nitrogen loss and also a shortened wound healing time in 16 severely burned adults treated with oxandrolone during their acute hospital stay, compared retrospectively with a historical cohort of 24 less severely burned subjects. 29 To discern both efficacy and cellular-level mechanism of improvement in a controlled clinical trial, we used an isotopic technique that enables quantification of the effect of oxandrolone on muscle protein and amino acid kinetics. Our results showed that oxandrolone stimulated muscle protein synthesis.
The amino acid precursors needed for enhanced muscle protein synthesis can come from either the blood or the process of muscle protein breakdown. Muscle protein synthesis efficiency can be considered the effectiveness of incorporation of the amino acid precursors from either source into protein. In the current study, oxandrolone did not alter inward transport, but it did cause an increase in protein synthesis efficiency. Oxandrolone treatment enhanced the reuse of free intracellular amino acids supplied from protein breakdown. Thus, protein synthesis was increased while protein breakdown was unaltered, thereby improving the net balance of muscle protein metabolism to a value that was statistically not different than zero.
The FSR of protein in muscle is determined by the net accumulation of labeled phenylalanine over time. This parameter is independent from the values derived from the three-compartment model calculations in that the three-compartment model does not rely on tissue incorporation data. In this study, there was a twofold increase in the FSR in patients receiving oxandrolone. This supports the notion that the mechanism for the improved net balance was due to a stimulation of muscle protein synthesis.
The stable isotope methodology and the three-compartment model used in this study allow delineation of physiologic fluxes of amino acids at the tissue and intracellular levels. The measured increases in our main outcome measures, net protein balance and FSR of muscle protein, corroborate with the positive clinical outcomes already reported by Demling. 28,29 By documenting oxandrolone’s efficacy and by delineating its physiologic mechanism of action in burn-induced catabolism, this study validates those clinical findings.
We found no serious adverse effects associated with oxandrolone treatment. Prothrombin times were normal during drug treatment. There were no differences in the rest of the liver function panels between oxandrolone and control patients. No patient progressed to clinically significant liver dysfunction. No induced hirsutism or other androgenic effects were noted. Finally, there were no differences in the incidence of sepsis or death between groups.
These protein kinetic results, coupled with the report of general clinical improvement by Demling, 28,29 strongly suggest that oxandrolone is an excellent choice for metabolic support of burn victims. In these three series, oxandrolone has improved outcome measures beyond what was possible in control patients already receiving optimal clinical burn treatment. Further, in addition to being safe, oxandrolone therapy is also inexpensive: its cost is only 10% of a comparable anabolic dose of recombinant human growth hormone. 29
Although we did select a specific subset of burn victims for this trial—nutritionally depleted, critically ill burned children in whom definitive surgical treatment had been delayed—these results should be applicable to a much wider spectrum of ill, catabolic patients. The results of a recent study by Sheffield-Moore et al 43 showed the same mechanism of action (improvement in muscle protein synthesis efficiency) in normal, nonburned, young adults. With efficacy and mechanism of action consistent in these two disparate groups, we propose that many other patients could benefit from this anabolic agent if they are suffering the complications of catabolism. Patients with pancreatitis, short-gut syndrome, or other types of severe trauma should be able to benefit from oxandrolone therapy.
In conclusion, we have shown that oxandrolone improves muscle protein kinetics in severely burned children. The mechanism by which this occurs is enhancement of muscle protein synthesis efficiency. This is a safe, inexpensive, and easily administered anabolic agent, and it is likely that it could benefit a wide range of surgical and medical patients.
Footnotes
Supported by SHC Grant #8660, SHC Grant #8490, NIH Training Grant #2T32GM0825611, NIH Center Grant #1P50GM60338-01, and NIH Grant #5RO1GM5729503.
Presented at the Society of Critical Care Medicine meeting in Orlando, Florida, February 12, 2000.
Correspondence: Dr. David N. Herndon, Shriners Hospitals for Children, 815 Market St., Galveston, TX 77550.
E-mail: dherndon@UTMB.edu
Accepted for publication August 28, 2000.
References
- 1.Wilmore D, Aulick L. Metabolic changes in burned patients. Surg Clin North Am 1978; 58: 1173–1280. [DOI] [PubMed] [Google Scholar]
- 2.Wilmore DW, Long JM, Mason AD Jr, et al. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg 1974; 180: 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newsome T, Mason A, Pruitt B. Weight loss following thermal injury. Ann Surg 1973; 178: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander JW. Nutrition and infection—A new perspective on an old problem. Arch Surg 1986; 121: 966–972. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe RR, Durkot MJ, Allsop JR, et al. Glucose metabolism in severely burned patients. Metabolism 1979; 28: 1031–1039. [DOI] [PubMed] [Google Scholar]
- 6.Bessey P, Jiang Z, Johnson D, et al. Post-traumatic skeletal muscle proteolysis: the role of the hormonal environment. World J Surg 1989; 13: 465–470. [DOI] [PubMed] [Google Scholar]
- 7.Wilmore DW, Mason AD, Johnson DW, et al. Effect of ambient temperature on heat production and heat loss in burn patients. J Appl Physiol 1975; 38: 593–597. [DOI] [PubMed] [Google Scholar]
- 8.Herndon DN, Parks DH. Comparison of serial debridement and autografting and early massive excision with cadaver skin overlay in the treatment of large burns in children. J Trauma 1986; 26: 149–152. [DOI] [PubMed] [Google Scholar]
- 9.Burke JF, Quinby WC, Bondoc CC. Early excision and prompt wound closure supplemented with immunosuppression. Surg Clin North Am 1978; 58: 1141–1150. [DOI] [PubMed] [Google Scholar]
- 10.Milner EA, Cioffi WG, Mason AD, et al. A longitudinal study of resting energy expenditure in thermally injured patients. J Trauma 1994; 37: 167–170. [DOI] [PubMed] [Google Scholar]
- 11.Klein GL, Herndon DN, Rutan TC, et al. Bone disease in burn patients. J Bone Mineral Res 1993; 8: 337–345. [DOI] [PubMed] [Google Scholar]
- 12.Klein GL, Herndon DN, Langman CB, et al. Long-term reduction in bone mass following severe burn injury in children. J Pediatr 1995; 126: 252–256. [DOI] [PubMed] [Google Scholar]
- 13.Rutan RL, Herndon DN. Growth delay in postburn pediatric patients. Arch Surg 1990; 125: 392–395. [DOI] [PubMed] [Google Scholar]
- 14.Wilmore DW, Moylan JA, Bristow BF, et al. Anabolic effects of human growth hormone and high caloric feedings following thermal injury. Surg Gynecol Obstet 1974; 138: 875–884. [PubMed] [Google Scholar]
- 15.Manson JM, Wilmore DW. Positive nitrogen balance with human growth hormone and hypocaloric intravenous feeding. Surgery 1986; 100: 188–197. [PubMed] [Google Scholar]
- 16.Gore DC, Honeycutt D, Jahoor F, et al. Effect of exogenous growth hormone on whole-body and isolated-limb protein kinetics in burned patients. Arch Surg 1991; 126: 38–43. [DOI] [PubMed] [Google Scholar]
- 17.Byme TA, Morrissey TB, Gatzen C, et al. Anabolic therapy with growth hormone accelerates protein gain in surgical patients requiring nutritional rehabilitation. Ann Surg 1993; 218: 400–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herndon DN, Barrow RE, Kunkel KR, et al. Effects of recombinant human growth hormone on donor-site healing in severely burned children. Ann Surg 1990; 2121: 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilpin DA, Barrow RE, Kunkel KR, et al. Recombinant human growth hormone accelerates wound healing in children with large cutaneous burns. Ann Surg 1994; 220: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herndon DN, Hawkins HK, Nguyen TT, et al. Characteristics of growth hormone enhanced donor site healing in patients with large cutaneous burns. Ann Surg 1995; 221: 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knox J, Demling R, Wilmore D, et al. Increased survival after major thermal injury: the effect of growth hormone therapy in adults. J Trauma 1995; 39: 526–531. [DOI] [PubMed] [Google Scholar]
- 22.Takala J, Ruokonen E, Webster NR, et al. Increased mortality with growth hormone treatment in critically ill adults. N Engl J Med 1999; 341: 785–792. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez RJ, Wolf SE, Barrow RE, et al. Growth hormone treatment in pediatric burns: a safe therapeutic approach. Ann Surg 1998; 228: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakurai Y, Aarsland A, Herndon DN, et al. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg 1995; 222: 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrando AA, Chinkes DL, Wolf SE, et al. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg 1999; 229: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X-J, Chinkes DL, Wolf SE, et al. Insulin but not growth hormone stimulates protein anabolism in skin wound and muscle. Am J Physiol (Endocrin Metab) 1999; 276: E712–720. [DOI] [PubMed] [Google Scholar]
- 27.Ferrando AA, Tipton KD, Doyle D, et al. Testosterone stimulates protein synthesis but not amino acid transport in humans. Am J Physiol (Endocrin Metab) 1998; 275: E864–871. [DOI] [PubMed] [Google Scholar]
- 28.Demling RH, DeSanti L. Oxandrolone, an anabolic steroid, significantly increases the rate of weight gain in the recovery phase after major burns. J Trauma 1997; 43: 47–51. [DOI] [PubMed] [Google Scholar]
- 29.Demling RH. Comparison of the anabolic effects and complications of human growth hormone and the testosterone analog, oxandrolone, after severe burn injury. Burns 1999; 25: 215–221. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson KO, Albertsson-Wikland K, Alm J, et al. Improved final height in girls with Turner’s syndrome treated with growth hormone and oxandrolone. J Clin Endocrin Metab 1996; 81: 635–640. [DOI] [PubMed] [Google Scholar]
- 31.Mendenhall CL, Moritz TE, Roselli GA, et al. A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: results of a Department of Veterans Affairs cooperative study. Hepatology 1993; 17: 564–570. [DOI] [PubMed] [Google Scholar]
- 32.Berger J, Poll L, Hall C, et al. Oxandrolone in AIDS-wasting myopathy. AIDS 1996; 10: 1657–1662. [DOI] [PubMed] [Google Scholar]
- 33.Fox M, Minor A, et al. Oxandrolone: a potent anabolic steroid. J Clin Endocrin Metab 1962; 22: 921–923. [DOI] [PubMed] [Google Scholar]
- 34.Karim A, Ranney RE, Zagarella BA, et al. Oxandrolone disposition and metabolism in man. Clin Pharmacol Ther 1973; 14: 862–866. [DOI] [PubMed] [Google Scholar]
- 35.Biolo G, Maggi SP, Williams BD, et al. Increased rates of muscle protein turnover and amino acid transport following resistance exercise in humans. Am J Physiol (Endocrin Metab) 1995; 268: E514–520. [DOI] [PubMed] [Google Scholar]
- 36.Wolfe RR. Radioactive and stable isotope tracers in biomedicine: principles and practice of kinetic analysis. New York: Wiley-Liss; 1992.
- 37.Pfeifer R, Karol R, Korpi J, et al. Practical application of HPLC to amino acid analysis. Am Lab 1983; : 78–87. [Google Scholar]
- 38.Biolo G, Chinkes D, Zhang X, et al. A new model to determine in vivo the relationship between amino acid transmembrane transport and protein kinetics in muscle. J Parenteral Enteral Nutr 1992; 16: 305–315. [DOI] [PubMed] [Google Scholar]
- 39.Jorfeld L, Waren J. Leg blood flow during exercise in man. Clin Sci Lond 1971; 41: 459–473. [DOI] [PubMed] [Google Scholar]
- 40.Mochizuki H, Trocki O, Dominioini L, et al. Mechanism of prevention of postburn hypermetabolism and catabolism by early enteral feeding. Ann Surg 1984; 200: 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mochizuki H, Trocki O, Dominioni L, et al. Reduction of postburn hypermetabolism by early enteral feeding. Curr Surg 1985; 42: 121–125. [PubMed] [Google Scholar]
- 42.Dominioni L, Trocki O, Fang CH, et al. Enteral feeding in burn hypermetabolism: nutritional and metabolic effects of different levels of calorie and protein intake. J Parenteral Enteral Nutr 1985; 9: 269–279. [DOI] [PubMed] [Google Scholar]
- 43.Sheffield-Moore M, Urban RJ, Wolf SE, et al. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrin Metab 1999; 84: 2705–2711. [DOI] [PubMed] [Google Scholar]