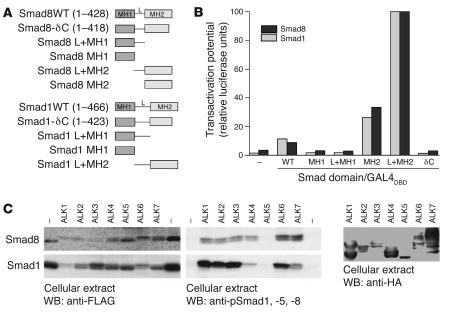
Figure 3. Smad8 is activated by TGF-β/BMP type I receptors.
(A) Schematic representation of WT Smads and the Smad domains used for functional studies in HEK 293T and C3H10T1/2 cells. MH1 and MH2 are the major conserved Smad domains; “L” shows the linker region between them. The linker region is considerably smaller in Smad8 than in Smad1. (B) Results of reporter assays in HEK 293T cells, in which a GAL4 reporter with the GAL4 DNA-binding domain (GAL4DBD) fused to various forms of Smad proteins was used. Results are expressed as relative luciferase units normalized to β-gal activity and presented as percent of Smad L+MH2, which was arbitrarily set as 100%. Pooled data from at least 3 independent experiments are presented. Smad8 MH2 and L+MH2 domains exhibited a constitutive active transactivating potential comparable to that of Smad1. (C) Activation potential of Smad8 and Smad1 signalling mediators by constitutively active TGF-β/BMP receptors (ALK1–ALK7) in HEK 293T cells. FLAG-tagged Smad8 or Smad1 was transiently coexpressed with constitutively active HA-tagged type I receptors. Expression of all type I receptors was mediated by the identical vector (pcDNA3). Expression rate of the receptors was determined by Western blotting using anti-HA antibodies. Receptor bands indicate variations in the glycosylation of the ectodomain. Type I receptor–dependent phosphorylation of Smads was shown by anti-pSmad1, -5, and -8 antibodies, which also react with phosphorylated Smad8. Smad1 and Smad8 were phosphorylated by most type I receptors. In contrast to Smad1, Smad8 was also efficiently phosphorylated by ALK4 and ALK7 (TGFβ1 receptors).