Abstract
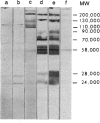
The differences were examined between IgE, IgG1 and IgG2a antibody responses against two kinds of nematode antigens in rats infected with Nippostrongylus brasiliensis. With ELISA studies, remarkable IgE and IgG1 antibody responses were observed against antigens in excretory/secretory products (ES) of N. brasiliensis, whereas the IgG2a antibody response against ES was negligible. On the other hand, antibody response to antigens in an extract of homogenized adult worm (AW) was observed mainly in IgG2a, with little response in IgE or IgG1. Immunohistochemical studies showed that IgE- and IgG1-binding antigens were localized almost exclusively in the subventral glands, a secretory apparatus in N. brasiliensis, while IgG2a-binding antigens were found mainly in the nematode wall along the body cavity. Immunoblot analysis revealed that the major IgE- and IgG1-binding molecules in ES were identical. On the other hand, some, but not all, of the major IgG2a-binding molecules in AW were different from the IgE/IgG1-binding molecules in ES. The findings suggest that the IgE/IgG1 and IgG2a antibody responses in N. brasiliensis-infected rats are induced by different groups of nematode antigens. Thus, it is presumed that the production of each class of antibody might be dependent, at least in part, on the nature of the antigen or antigen-linked molecules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Diaz-Sanchez D., Kemeny D. M. Generation of a long-lived IgE response in high and low responder strains of rat by co-administration of ricin and antigen. Immunology. 1991 Feb;72(2):297–303. [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez D., Kemeny D. M. The sensitivity of rat CD8+ and CD4+ T cells to ricin in vivo and in vitro and their relationship to IgE regulation. Immunology. 1990 Jan;69(1):71–77. [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Holmes J., Ohara J., Tung A. S., Sample J. V., Paul W. E. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988 Oct 1;141(7):2335–2341. [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Snapper C. M., Ohara J., Paul W. E. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong T. A., Mosmann T. R. Alloreactive murine CD8+ T cell clones secrete the Th1 pattern of cytokines. J Immunol. 1990 Mar 1;144(5):1744–1752. [PubMed] [Google Scholar]
- HayGlass K. T., Stefura B. P. Anti-interferon gamma treatment blocks the ability of glutaraldehyde-polymerized allergens to inhibit specific IgE responses. J Exp Med. 1991 Feb 1;173(2):279–285. doi: 10.1084/jem.173.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayglass K. T., Gieni R. S., Stefura W. P. Long-lived reciprocal regulation of antigen-specific IgE and IgG2a responses in mice treated with glutaraldehyde-polymerized ovalbumin. Immunology. 1991 Aug;73(4):407–414. [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Miller H. R. Production and activities of IgE in helminth infection. Prog Allergy. 1982;31:178–233. [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Pearce E. J., Caspar P., Grzych J. M., Lewis F. A., Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991 Jan 1;173(1):159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond L., Wassom D. L., Hayes C. E. Evidence for differential induction of helper T cell subsets during Trichinella spiralis infection. J Immunol. 1989 Dec 15;143(12):4232–4237. [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Bonnefoy J. Y., Spits H., Yokota T., Arai N., Arai K., Banchereau J. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6880–6884. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Paliard X., Banchereau J., Spits H., De Vries J. E. IgE production by normal human B cells induced by alloreactive T cell clones is mediated by IL-4 and suppressed by IFN-gamma. J Immunol. 1988 Aug 15;141(4):1218–1224. [PubMed] [Google Scholar]
- Sher A., Coffman R. L., Hieny S., Cheever A. W. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J Immunol. 1990 Dec 1;145(11):3911–3916. [PubMed] [Google Scholar]
- Snapper C. M., Peschel C., Paul W. E. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J Immunol. 1988 Apr 1;140(7):2121–2127. [PubMed] [Google Scholar]
- Snapper C. M., Peçanha L. M., Levine A. D., Mond J. J. IgE class switching is critically dependent upon the nature of the B cell activator, in addition to the presence of IL-4. J Immunol. 1991 Aug 15;147(4):1163–1170. [PubMed] [Google Scholar]
- Soloway P., Fish S., Passmore H., Gefter M., Coffee R., Manser T. Regulation of the immune response to peptide antigens: differential induction of immediate-type hypersensitivity and T cell proliferation due to changes in either peptide structure or major histocompatibility complex haplotype. J Exp Med. 1991 Oct 1;174(4):847–858. doi: 10.1084/jem.174.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Tada T., Okumura K., Taniguchi M. Regulation of homocytotropic antibody formation in the rat. VII. Carrier functions in the anti-hapten homocytotropic antibody response. J Immunol. 1972 Jun;108(6):1535–1541. [PubMed] [Google Scholar]
- Takatsu K., Ishizaka K. Reaginic antibody formation in the mouse. VII. Depression of the ongoing IgE antibody formation by suppressor T cells. J Immunol. 1976 Oct;117(4):1211–1218. [PubMed] [Google Scholar]
- Urban J. F., Jr, Katona I. M., Paul W. E., Finkelman F. D. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Amano J., Aicher W. K., Taguchi T., Kiyono H., McGhee J. R. Selective induction of Th2 cells in murine Peyer's patches by oral immunization. Int Immunol. 1992 Apr;4(4):433–445. doi: 10.1093/intimm/4.4.433. [DOI] [PubMed] [Google Scholar]
- Yamada M., Nakazawa M., Matsumoto Y., Arizono N. IgE antibody production in rats against multiple components of excretory-secretory products of the nematode Nippostrongylus brasiliensis. Immunology. 1991 Jan;72(1):104–108. [PMC free article] [PubMed] [Google Scholar]