Abstract
Objective
To examine the effect of an integrated surgical approach to the treatment of acute type A dissections.
Summary Background Data
Acute type A dissection requires surgery to prevent death from proximal aortic rupture or malperfusion. Most series of the past decade have reported a death rate in the range of 15% to 30%.
Methods
From January 1994 to March 2001, 104 consecutive patients underwent repair of acute type A dissection. All had an integrated operative management as follows: intraoperative transesophageal echocardiography; hypothermic circulatory arrest (HCA) with retrograde cerebral perfusion (RCP) to replace the aortic arch; HCA established after 5 minutes of electroencephalographic (EEG) silence in neuromonitored patients (66%) or after 45 minutes of cooling in patients who were not neuromonitored (34%); reinforcement of the residual arch tissue with a Teflon felt “neo-media”; cannulation of the arch graft to reestablish cardiopulmonary bypass at the completion of HCA (antegrade graft perfusion); and remodeling of the sinus of Valsalva segments with Teflon felt “neo-media” and aortic valve resuspension (78%) or replacement with a biologic or mechanical valved conduit (22%).
Results
Mean age was 59 ± 15 (range 22–86) years, with 71% men and 13% redo sternotomy after a previous cardiac procedure. Mean cardiopulmonary bypass time was 196 ± 50 minutes. Mean HCA with RCP time was 42 ± 12 minutes (range 19–84). Mean cardiac ischemic time was 140 ± 45 minutes. Eleven percent of patients presented with a preoperative neurologic deficit, and 5% developed a new cerebrovascular accident after dissection repair. The in-hospital death rate was 9%. Excluding the patients who presented neurologically unresponsive or with ongoing cardiopulmonary resuscitation (n = 5), the death rate was 4%. In six patients adverse cerebral outcomes were potentially avoided when immediate surgical fenestration was prompted by a sudden change in the EEG during cooling. Forty-five percent of neuromonitored patients required greater than 30 minutes to achieve EEG silence.
Conclusion
The authors have shown that the surgical integration of sinus segment repair or aortic root replacement, the use of EEG monitoring, partial or total arch replacement using RCP, routine antegrade graft perfusion, and the uniform use of transesophageal echocardiography substantially decrease the death and complication rates of acute type A dissection repair.
Aortic dissection is the most common acute aortic catastrophe requiring emergent surgical therapy. 1 Urgent surgical treatment of acute type A dissection is mandated because medical treatment is associated with a 60% in-hospital death rate. 2 Since the first description of the successful repair of an aortic arch aneurysm by DeBakey et al 3 in 1955, numerous advances have been described in the diagnosis and management of thoracic aortic diseases. 4,5
Despite these advances, acute aortic dissection remains one of the most challenging operations to the cardiovascular surgeon. During the past decade, there have been numerous reports describing surgical results after repair of acute type A dissection. These data reveal a perioperative death rate of 20% to 30%. 6–8 The recent report from the multiinstitutional International Registry of Acute Aortic Dissection 2 noted a 26% death rate in surgically treated patients with type A dissection despite the relatively high volume of aortic surgery in the centers participating in the study.
The purpose of this study was to examine the effect of a standardized surgical and circulation management approach to the clinical outcome in acute type A dissections at our institution. Whereas many of the recent advances have focused on individual facets that make up the complex repair of type A dissections, our emphasis has been to integrate many of these advances into a standardized preoperative and operative management of this disease. This approach includes using continuous intraoperative transesophageal echocardiography (TEE), hypothermic circulatory arrest (HCA) with retrograde cerebral perfusion (RCP), neurocerebral monitoring whenever possible, resection of most or all of the arch with reinforcement of the distal aorta using either felt or BioGlue as a neo-media, cannulation of the arch graft to reestablish antegrade graft perfusion at the completion of the aortic arch reconstruction, and reconstruction of the native aortic root when feasible with aortic valve resuspension and felt reinforcement of the sinus segments with felt as neo-media. TEE was used to confirm the diagnosis, to assess the aortic valve both before and after repair, and to monitor the status of brachiocephalic malperfusion and intraoperative dynamic circulatory changes secondary to the initiation of cardiopulmonary bypass (CPB).
METHODS
Patients and Methods
A retrospective review was performed of 104 consecutive patients from January 1994 to March 2001 undergoing surgical repair of acute type A dissections using an institutional standardized therapeutic protocol. All patients studied received the surgical and circulation management approach as described in the introduction. Follow-up data were obtained from review of patient charts.
Demographics
Mean age was 59 ± 15 years (range 22–86). There were 74 men and 30 women (Table 1). The preoperative diagnosis of a dissection was documented by magnetic resonance imaging, computed tomography, or TEE. All patients had an intraoperative TEE to confirm the diagnosis. Eleven patients had a preoperative stroke, 11 patients were hemodynamically unstable, and 13 patients had previous cardiac surgery (Tables 2 and 3). Unstable patients were defined those with systolic blood pressure less than 70 mmHg, requirement for preincision cardiopulmonary resuscitation (CPR), or free ascending aortic rupture after opening of the pericardium. All preoperative strokes were secondary to dissection-related brachiocephalic malperfusion syndromes. All patients with an obtainable blood pressure were included in the study regardless of preoperative malperfusion or neurologic deficit. Patients with superior mesenteric artery occlusion, cadaveric extremities, or metabolic acidosis were included. Patients requiring external CPR for longer than 20 minutes before skin incision were excluded.
Table 1. DEMOGRAPHICS
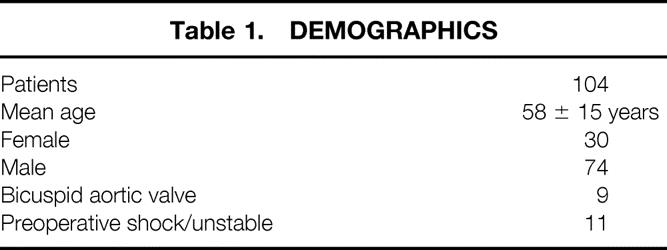
Table 2. OPERATIVE DATA
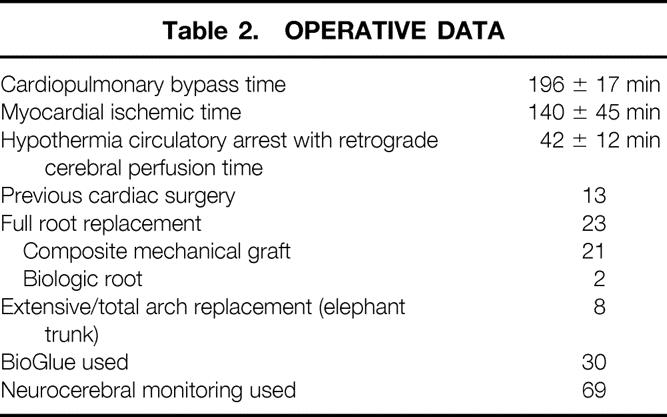
Table 3. NEUROCEREBRAL DATA
EEG, electroencephalographic.
All patients were monitored for focal cerebrovascular accidents (CVA) and temporary neurologic deficits (TND). CVA was defined as a clinically evident and/or radiologically documented focal deficit. TND was defined as a clinically evident nonfocal neurologic dysfunction as determined by a neurologist.
Surgical Technique
After endotracheal intubation, a TEE probe was inserted and continuous electroencephalographic (EEG) monitoring was initiated. Standard antegrade and retrograde cold blood cardioplegia was used for cardiac protection. The CPB and RCP circuit used has been described previously. 9 Briefly, venous cannulation consisted of a double-stage right atrial venous cannula connected by a Y connector to a small right-angle single-stage superior vena cava cannula. During HCA, oxygenated blood was infused via the snared superior vena cava cannula using either an arteriovenous bridge or the cardioplegia line connected to the venous Y. In most patients, the femoral artery was used for arterial cannulation. Occasionally, the right axillary/subclavian artery was used for primary arterial cannulation. In 69 patients, neurocerebral monitoring was performed with the use of EEG monitoring during the entire operation. Neuromonitored patients were cooled for 5 minutes beyond EEG silence and 3°C below EEG silence, which usually occurred at a nasopharyngeal temperature between 15° and 20°C. 10 Details of our neurocerebral protocols have been previously described. 11,12 When neurocerebral monitoring was not available, patients were cooled for a total of 45 minutes. The time of 45 minutes was chosen after preliminary data on neurocerebral monitoring in aortic arch aneurysm repairs showed that most patients achieved EEG silence after 45 minutes of active cooling. The ascending aorta was not clamped during the cooling period except when aortic valve insufficiency mandated ascending aorta clamping to prevent left ventricular dilatation. All patients had a left ventricular vent inserted via the right superior pulmonary vein.
Once profound hypothermia was achieved, antegrade CPB was interrupted and oxygenated blood at 12°C was infused into the superior vena cava cannula, which was snared between the azygous vein and the right atrial junction. With the patient in slight Trendelenburg position, an RCP perfusion pressure of 25 mmHg was maintained, yielding a flow between 200 and 300 mL/min. The innominate vein was inspected to confirm bilateral jugular venous system inflow. Throughout the RCP period, dark blood emanated from the brachiocephalic arterial orifices, implying oxygen extraction.
Resection of the aortic arch and ascending aorta was undertaken and a Dacron tube graft was used as a replacement conduit. Felt or BioGlue was placed between the intima and adventitia to obliterate the false lumen and recreate a neo-media (Fig. 1). Hemiarch repair was used in 96 patients, and an extensive or total aortic arch replacement (elephant trunk procedure) was used in 8 patients. The primary tear site was resected in all patients. On completion of the aortic arch reconstruction, blood was allowed to occupy the native aorta and graft, allowing air and debris to be evacuated from the cerebrovascular system. The entire arterial circulation was deaired at this time via the RCP circuit. The arch graft was cannulated and then proximally cross-clamped, with RCP termination and resumption of arterial perfusion and rewarming directly through the aortic arch graft for antegrade perfusion.
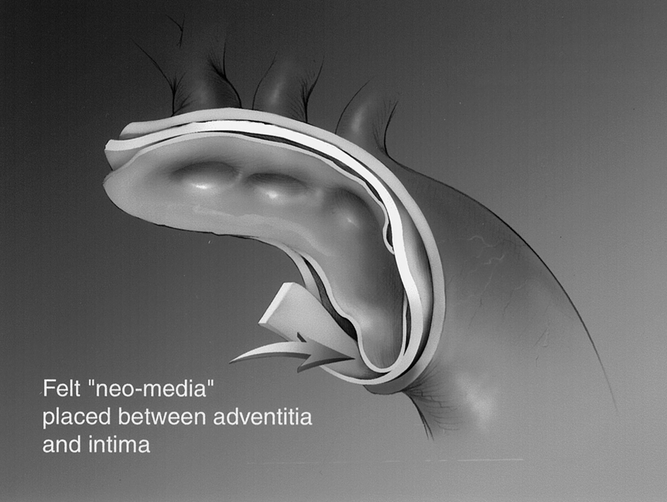
Figure 1. Application of felt “neo-media” placed between adventitia and intima.
The aortic root was replaced or repaired depending on the pathology present. When repair was deemed possible, the aortic valve leaflets were resuspended using three pledgeted supracommissural sutures. The sinus of Valsalva segments were then reinforced with Teflon felt as a neo-media (Fig. 2), and more recently BioGlue was used as an adjunct. In 81 patients, the aortic root was repaired, and in 23 patients the aortic root was replaced with either a biologic or a mechanical valved conduit (see Table 2). Indications for the replacement of the aortic root included bicuspid aortic valve (n = 9), Marfan syndrome (n = 10), Ehlers-Danlos syndrome (n = 1), primary abnormalities of the aortic valve leaflets, obvious sinus of Valsalva aneurysm, and extension of both the tear and dissection to the aortic annulus (see Table 2). TEE was used in all patients to assess the adequacy of the aortic root repair.
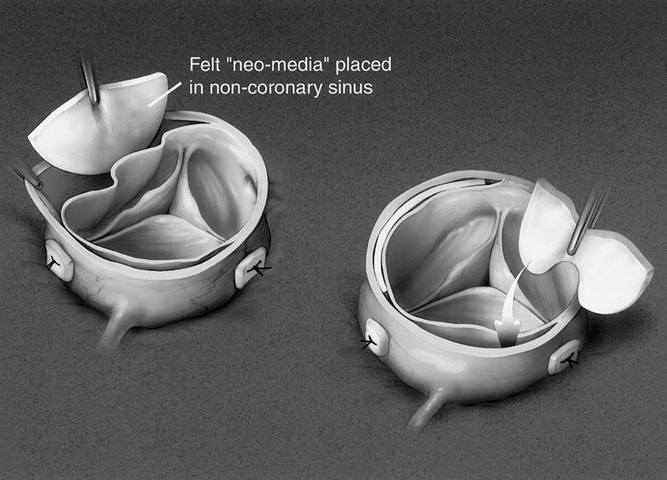
Figure 2. Application of felt “neo-intima” placed in non-coronary sinus.
Pharmacologic Adjuncts
Routine neuroprotective pharmacology was used in all patients according to the following protocol. All patients received 1 g methylprednisolone (Solu-Medrol) intravenous bolus, 1 g MgS04 intravenous, 2.5 mg/kg lidocaine intravenous, and 12.5 g mannitol intravenous. These neuroprotective agents were given immediately before initiation of CPB.
Statistical Analysis
All variables are expressed as mean ± standard deviation of the mean. Significance was determined by unpaired t test analysis.
RESULTS
Surgical Data
Intraoperative data are shown in Table 2. Mean CPB time was 196 ± 50 minutes. Mean HCA/RCP time was 42 ± 12 minutes. Mean cardiac ischemic time was 137 ± 14 minutes. Hemiarch reconstruction was used in 92% of patients (96/104) and extensive or total arch replacement in 8% (8/104). Aortic root repair was performed in 81 patients (78%) and biologic or mechanical valved conduits in 22% (23/104). BioGlue has recently been added to our reconstructive armamentarium and was used in 31 patients to facilitate the obliteration of the false lumen at both the aortic root and the distal aortic arch. Thirteen percent (13/104) of patients had previous cardiac surgery.
Neurocerebral Monitoring
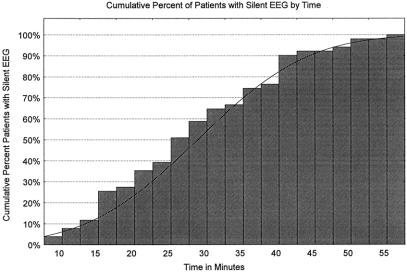
Intraoperative monitoring (EEG) was used in 65% (65/104) of patients. Mean time to EEG silence was 29 ± 17 minutes. Forty-five percent of patients required greater than 30 minutes of active cooling to achieve EEG silence, and 10% of neuromonitored patients required longer than 45 minutes of cooling time to become electrically silent on EEG monitoring (Fig. 3). The 36 patients who did not undergo neurocerebral monitoring were all cooled for 45 minutes. 11,12
Figure 3. Time to EEG silence.
Neurocerebral Monitoring and Circulation Management
In neuromonitored patients, HCA and RCP are initiated based on EEG silence and not on any absolute temperature or cooling time. Intraoperative circulation management was changed in six patients (6%) when EEG changes were detected after initiation of extracorporeal circulation (see Table 3). These changes were due to malperfusion of arch branches leading to lack of adequate cerebral blood flow. These immediate EEG findings were followed by prompt surgical therapy consisting of cannulation site changes (n = 1) or cross-clamp release with aortic arch fenestration (n = 5). Potential postoperative CVAs were avoided in this patient population.
Neurologic Outcomes
Eleven patients (11%) presented with a preoperative stroke. Five patients (5%) developed a new focal stroke after surgery. TND occurred in 10 patients (9.6%). Mean HCA/RCP time was 55 ± 13 minutes in patients who developed TND versus 40 ± 11 minutes in patients without neurologic dysfunction (P = .004). Thirty percent (6/20) of patients with HCA/RCP times greater than 50 minutes had TND, whereas only 4.7% (4/84) patients with HCA/RCP times less than 50 minutes had TND.
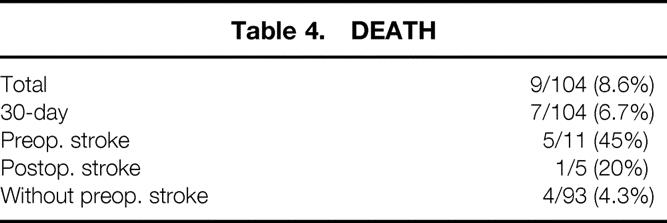
Death
The in-hospital perioperative death rate was 9% (n = 9). The 30-day perioperative death rate was 7% (n = 7) (Table 4). Five patients (5%) were pronounced brain dead from significant preoperative neurologic injury shortly after successful and uneventful proximal thoracic aorta reconstruction. Four additional patients died after surgery. One patient died secondary to postoperative malperfusion to his colon, one patient died of a postoperative gastrointestinal hemorrhage, one patient died from a Pseudomonas fasciitis of malperfused lower extremities, and one patient died of multiple organ failure. If patients who presented neurologically unresponsive or with ongoing CPR are excluded (n = 5), the operative death rate decreases to 4% (n = 4).
Table 4. DEATH
Relationship of Stroke to Death
Forty-five percent (5/11) of patients presenting with type A dissection and preoperative CVA died. All four patients who presented neurologically unresponsive and intubated died despite successful proximal aortic reconstruction. Twenty percent (1/5) of those sustaining a postoperative CVA died.
DISCUSSION
Acute type A dissection is a surgical disease. Early reports indicated a 90% 1-month death rate for patients with type A dissection treated without surgery. 13–15 More recent publications documenting medical management of acute type A dissection, with newer antihypertensive medications, have reported a 60% 1-month death rate. 2
Since the first successful type A dissection repair by Morris et al 16 in 1963, substantial improvements have been made in intraoperative cardiac and cerebral protection, management of the aortic root and valve, and prosthetic materials. After the introduction of hypothermic circulatory arrest for aortic arch reconstruction, 17,18 Livesay et al 4 first advocated the routine use of an open distal anastomosis during the repair of acute type A dissection. Subsequent reports outlined the decision-making algorithms required for reconstruction of the dissected aortic root. 7 As surgical experience with acute type A dissection increased, surgeons better understood which patients would be better served with valve-sparing aortic root repairs and which pathology required full aortic root replacement. In addition, newer diagnostic modalities such as TEE, computed tomography, and magnetic resonance imaging improved diagnostic accuracy compared with invasive angiography. Certainly the introduction of collagen-impregnated grafts improved the outcomes, because surgical repair of type A dissection had been associated in the past with postoperative exsanguination. Circulation management and intraoperative cerebral protection further improved with the introduction of retrograde cerebral perfusion as an adjunct to HCA during an open aortic arch. 5,6,19–21 Most recently, postrepair antegrade arch graft perfusion and the use of axillary or subclavian artery cannulation techniques 8 may contribute to decrease the incidence of perioperative malperfusions. 22,23
Despite these advances in the treatment of acute type A dissection, many recent reports continue to show high death rates 2,24,25 of approximately 25%. This study shows that a routine integrated surgical strategy incorporating recent diagnostic and therapeutic advances improves outcomes in this complex cardiovascular catastrophe.
It is our belief, shared by some others, 26 that neurocerebral monitoring can improve outcomes for multiple reasons. EEG silence, as opposed to an absolute brain temperature or an absolute cooling time, is the optimal marker for minimal cerebral metabolic activity, thus leading to improved cerebral protection when HCA is conducted under EEG silence. In addition, the use of neurocerebral monitoring during surgery can potentially improve circulation management strategy. Our data show that only 46% of patients were EEG silent after 30 minutes of cooling; moreover, 10% of patients were still EEG active after 45 minutes of cooling. At the opposite spectrum, neurocerebral monitoring for many patients allows safe termination of cooling at EEG silence, often shortening both the cooling and subsequent rewarming phase. Without neurocerebral monitoring, we have been actively cooling all patients for 45 minutes, because 30 minutes does not even ensure EEG silence in most patients.
Our data corroborate data by Kleine et al 27 revealing a 5% to 10% incidence of malperfusion after establishment of CPB and/or cardiac fibrillation in acute type A dissection. These malperfusion incidents were immediately identified with neurocerebral monitoring and were subsequently addressed in an expeditious fashion by inflow cannulas reposition or direct surgical fenestration.
Our data revealed a 100% death rate in patients presenting neurologically unresponsive and intubated secondary to arch vessel malperfusion. In addition, we noted a 45% death rate in all patients presenting with a CVA who subsequently had an otherwise successful type A dissection repair. These data suggest a role for initial medical management in this high-risk group. Selective nonoperative management has been proposed in several recent clinical series. 2,28 Conversely, our data show that patients presenting neurologically intact have only a 4% postoperative death rate, suggesting that expeditious repair is warranted and highly efficacious using our approach.
We advocate the routine use of an open aortic arch with aggressive hemiarch reconstruction (see Fig. 1). In our series 8% of patients with type A dissections required a total arch replacement with an elephant trunk technique. The remainder (92%) underwent a relatively aggressive hemiarch repair. This technique allows for routine inspection of the aortic arch vessels with excision of most of the diseased aortic arch, followed by a precise reconstruction of the friable distal aorta with a single arch anastomosis. Subsequent cannulation of the arch graft for antegrade graft perfusion is then performed with resumption of CPB 1 and rewarming. While an open arch technique probably reduces the incidence of perioperative CVA and death compared with aortic clamping, 6,8 we hope that this strategy will improve the freedom from distal aortic reoperation in our patients.
Our preferred circulation management technique during open aortic arch repair consists of HCA with RCP. Several clinical series have described the successful use of RCP during aortic arch and type A dissection procedures, many of which have noted decreased stroke rates. 6,9,19,20,21,29–35 However, our TND rate of 10% may indicate that no cerebral protection method may be completely adequate at HCA times of greater than 50 minutes. Our data show that patients with a postoperative TND had significantly higher HCA/RCP times. It is well known that TND is a marker of global cerebral ischemia. An antegrade selective cerebral perfusion method may improve our results with prolonged HCA times. However, our data also revealed a low postoperative focal stroke rate, suggesting that RCP may play a role in decreasing debris, air, or other embolic phenomena.
In this series 78% of all patients with acute type A dissection underwent native aortic valve-sparing root repair. Most patients with dissections have fundamentally normal aortic valve architecture that has been disrupted by the dissection process propagating to the aortic annulus, so it makes sense to retain these otherwise normal valves whenever possible. Our indications for aortic root replacement included Marfan syndrome, obvious sinus of Valsalva aneurysm, bicuspid valve or other primary leaflet pathology, and when both the dissection and tear extend proximally to the aortic annulus. Otherwise we try to resuspend all aortic valves with repair of the dissected sinus segments.
Increasingly, we have been using the operating room as both a diagnostic and therapeutic suite, with direct admission from the helipad or emergency room to the operating room as often as possible. It is well known that the natural history of acute type A dissection has a high hourly death rate. This preoperative management strategy allows a reduction in the interval between diagnosis and therapy. Intraoperative TEE can then either confirm or establish the primary diagnosis of acute type A dissection.
In conclusion, our data show improved survival and low postoperative stroke rates with the use of an integrated perioperative approach to acute type A dissection. Together, these measures create a new paradigm that consists of:
Rapid admission to the operating room for diagnosis and therapy
Intraoperative TEE
Neurocerebral monitoring
Routine open aortic arch reconstruction with RCP
Routine antegrade arch graft perfusion after completion of arch repair
Aortic root repair and aortic valve resuspension in most patients when preexisting leaflet or root pathology is absent
Creation of a neo-media using either felt or BioGlue to strengthen the aortic and sinus walls and obliterate the false lumen.
DISCUSSION
Dr. William A. Baumgartner (Baltimore, Maryland): Dr. Bavaria and his co-authors are to be congratulated on their results in the management of type A dissection.
The authors have reported on 104 consecutive patients who underwent repair of acute type A dissection. In-hospital mortality was 9%, which included five patients who were neurologically unresponsive or undergoing CPR on arrival in the operating room. Only 5% exhibited postoperative stroke. The authors attribute these outstanding results to a number of changes they made in the overall management of this high risk group of patients. These included direct transfer to the operating room, a diagnosis made by transesophageal echocardiography, the use of neurocerebral monitoring to determine the time of cooling and routine open artery construction with retrograde cerebral perfusion. These methods, along with other standard principles, as well as excellent surgical technique, led to these good outcomes. The apparent operative mortality of 9% in this series of patients is one of the more impressive elements of the paper. A number of studies of acute type A dissection report perioperative mortality between 20 and 30%. The Journal of the American Medical Association recently published a multi-institutional international registry of acute aortic dissection revealing a 26% perioperative mortality. The authors have used neurocerebral monitoring as the basis for cooling their patients prior to hypothermic circulatory arrest. In six patients these EEG changes resulted in an urgent change in operative technique, suggesting that these patients were spared postoperative neurologic injury.
The use of EEG monitoring in patients undergoing hypothermic circulatory arrest was advocated by the late Dr. Stanley Crawford. In his book he said, “The temperature level which is both safe and protective in these cases is not known, but the level which produces dilated pupils after EEG is considered minimal.” In regard to strategy, Dr. Bavaria, did you use alpha stat or pH stat monitoring, and do you think this makes a difference?
Your mortality was significantly influenced by five of the nine patients whom you took to the OR who were either neurologically unresponsive or undergoing cardiopulmonary resuscitation. In Dr. Gott’s recent lecture at the American Heart Association he reported on the Hopkins, Marfan experience in which 36 of the 271 patients who underwent aortic root replacement were taken to the OR on an urgent or emergent basis. The only two individuals who died in this
entire group of 271 patients arrived moribund in the operating room with ongoing CPR. In view of your findings, would you change your strategy regarding this group of gravely ill patients?
Dissection of the aorta and aortic aneurysm surgery are some of the most difficult procedures encountered by adult cardiac surgeons. This slide that I borrowed from Dr. Gott is a quote from Sir William Osler: “There is no disease more conducive to clinical humility than aneurysm of the aorta.”
The authors have combined a number of recent advances into a systematic and standard approach to both the preoperative and operative management of this disease. These steps in combination with very good surgical technique have resulted in excellent outcomes and I believe set a new standard for the operative treatment of patients with acute type A dissection.
Presenter Dr. Joseph E. Bavaria (Philadelphia, Pennsylvania): Thank you, Dr. Baumgartner.
Regarding your questions about alpha stat and pH stat, we use the standard at our institution which is non-temperature corrected alpha stat management.
As far as CPR on the way to the operating room, I do not believe that a patient who is undergoing active CPR and proceeds to an arch procedure plus an ascending aortic procedure, can survive. So I think if a patient is having ongoing CPR they probably aren’t going to survive and you probably shouldn’t do the operation.
For neurologically unresponsive patients, I presented in the data our position, which is: we should probably wait on those patients to see how they do. We have had a couple of patients presenting neurologically unresponsive and we did not operate because they were obviously brain dead or close to brain dead.
Dr. W. Randolph Chitwood, Jr. (Greenville, North Carolina): I would like to congratulate Dr. Bavaria and his co-authors for what I believe is a very important paper on this subject. This is the kind of work that we have grown to expect from this superb cardiac surgical group at the University of Pennsylvania, and especially Dr. Bavaria, who is leading this aneurysm team.
In 104 patients with these very difficult aneurysms that we all fear in the middle of the night, they have achieved excellent repair results. As Dr. Baumgartner mentioned, the hospital mortality was only 9%, compared to many other series which show between 20 and 30% mortality. And this is without those who were salvage procedures, patients undergoing CPR and all types of problems that the patients that we have seen usually don’t survive. The mortality if you exclude those patients was only 4%, which I think is amazing.
Moreover, neurologic complications were very acute. I believe with the technique of deep hypothermic circulatory rest with the rest intervals based on cerebral activity, as you have shown, and quiescence by EEG and not temperature is important is really the seminal message of your paper. Moreover, retrograde cerebral perfusion probably added a great deal. And most of us use this technique. However, other studies with retrograde cerebral perfusion did not show this level of neurologic safety.
Electrical response is probably the key, and I think we all carry this away. When we think we are cooling sufficiently, we may or may not be doing this. I have several questions for you.
How do you handle patients who are at the greatest risk of cerebral malperfusion from the combination of aortic dissection, peripheral arteries, and cardiopulmonary bypass? Do you use the subclavian artery in those instances? How do you handle those?
Have you studied the cerebral effluent metabolically, the effluent coming from the arteries during retrograde cerebral perfusion, and compared this to temperature and EEC measures? This would be a very scientific and interesting study.
Use bioglue — which has only been introduced in the last several years in this country. Please describe the different bio-adhesive options that we have today in the United States, and tell us the status of GRF, the European glue, or the French glue. Have you ever just glued the walls together without suturing the valve and resuspending the valve?
And my last question. You found the best way to rapidly transport. In your manuscript you talk about rapid helicopter transport, with patients going directly to the operating room. Have you abandoned arteriography study in these patients in lieu of transesophageal echos to tell you where the dissections enter and reenter?
Dr. Joseph E. Bavaria: Thank you very much, Dr. Chitwood. To answer your questions:
Regarding retrograde cerebral perfusion and temporary neurological dysfunction: I completely agree with you regarding the fact that EEG silence is key. We found temporary neurological dysfunction, or global dysfunction, to be dependent on the time of the arch operation. The average HCA/RCP time for a TND patient was 55 minutes whereas the average time for the cohort in general was 39 minutes. And that finding was statistically significant.
Regarding cerebral malperfusion and circulation management strategies, we do use axillary/subclavian cannulation selectively. We also changed to subclavian cannulation on some cases when the EEG acutely changed after initiation of CPB.
Regarding the effluent through the arch during RCP, unfortunately we have not studied this significantly.
We use bioglue routinely now. I was involved in the prospective randomized IDE study in the United States, and ever since we finished that study we have been using bioglue on every Type A dissection repair. We use it as a “neo-media” and then coat the anastomosis after it is completed. Be very, very careful at the root, because the glue will turn into leather, which is good for an aortic dissection but not as good in the coronary arteries.
Regarding rapid transport, we believe in this concept, and we have taken lessons from our trauma team using helicopter transport directly to the operating room. We use the OR as both a therapeutic and diagnostic suite.
Primary arteriography has been completely abandoned. We never use it. The only time we ever see a dissection diagnosed by arteriography is when it is diagnosed in the cardiac cath lab by mistake.
Dr. Frank C. Spencer (New York, New York): Several of my comments have already been emphasized by previous discussants. This paper is especially significant because it may be the lowest mortality reported with acute aortic dissections. As the authors stated, it decreased from 25% before 1994 to the present low level, an especially low level if the patient did not have a preoperative stroke. I couldn’t tell from the presentation, which emphasizes several principles, about what was primarily responsible for this impressive decrease. I assume with the manuscript that some of the needed numerical data will be provided. I have several questions.
How rapid is “Rapidly to the O.R.”? You mentioned that angiography was not done. This leads to the fearsome possibility of urgently opening the chest for the wrong reason. Has this happened? Are patients literally rushed to the operating room like a rupture of an abdominal aneurysm or a stab wound or are they given 1, 6, 12 hours of evaluation and resuscitation? Is the diagnosis completely by TE echo in the O.R.? If so, what is the frequency of false positives and negatives?
A separate line of questions concerns ischemia time. 140 minutes myocardial ischemia was listed in the abstract. I believe you described temporary cerebral dysfunction with 55 minutes of ischemia but very little with 30 minutes. Is that related to retrograde perfusion? Is retrograde perfusion used in everybody; how much for how long?
Finally, with this impressive low mortality, what is your technique of myocardial preservation? 140 minutes of aortic occlusion represents a lot of ischemia time.
In terms of late results, any recurrences? Has there been any infection with the glue?
I have these many questions because as stated earlier, this is a most impressive report. My compliments to all of you.
Dr. Joseph E. Bavaria: Thank you very much, Dr. Spencer.
First of all regarding the low mortality rate. Actually, Tirone David presented a paper that was published I believe in 2000, presented in 1999, which showed a similar mortality rate. It was 9 or 10% as well. And it was a somewhat similar approach as he changed his algorithm from the late ’80s and early ’90s to something different in the late ’90s.
Regarding how rapid is rapid; we try to admit everybody who has a type A dissection diagnosis to the operating room. We don’t do anything more there than just put them on the table and then do a TEE to confirm the diagnosis. Most of the time these patients will have either a CT scan or another TEE. Our intraoperative TEE is usually just a confirmation.
I would say, however, about 5% of the time we will take a patient to the OR, we will put them to sleep, and we will do the TEE, and discover the outside hospital diagnosis is wrong and we then just wake them up in the intensive care unit. But, basically, by direct admission to the OR, we completely eliminate the entire “fire drill” in the ICU, the elevator ride, et cetera. So our protocol is nothing more skipping an ICU step and making the process more efficient.
TEE is 95% confirmatory, although in 5% of cases, we have made a different diagnosis. I can remember one case where we had a type A dissection and a 10% LVEF. When the TEE showed a 10% EF, we decided to treat him medically as he had no AI. Only one patient in seven years had a TEE misdiagnosis, and that was a ruptured type B dissection, that our echocardiographers felt was a type A dissection.
Myocardial preservation is simply not a problem in our experience. We use antegrade and retrograde cardioplegia. These patients are very cold and cardiac function is simply not an issue unless there is a preoperative myocranial infarction related to coronary malperfusion.
Regarding our late results, we had one infection of a graft that we noticed at four days. We took him back to the operating room, wrapped the entire aorta and graft with omentum, and he survived. Late results at the root and distal aorta, i.e., freedom from late reoperation at the distal aorta and freedom from late reoperation on the proximal aorta; we simply don’t have any really solid data yet. I don’t know whether our rates of late reoperation are better or worse than they were before institution of this protocol.
Retrograde cerebral perfusion, how much, and the issue of ischemic times. We have two important ischemic times, cerebral ischemic time during circulatory arrest and myocardial ischemic time average. Myocardial ischemic time is about 140 minutes. Cerebral ischemic time averaged about 40 minutes.
Dr. Hassam Najafi (Chicago): I would like to join the other discussants and extend my compliments to you and your colleagues for this excellent paper and particularly for the wonderful results you have achieved in this very difficult group of patients.
The only reason I come to the podium is to caution against saying that the patient who has cardiac arrest probably should not be operated on. I think it depends on where the cardiac arrest occurs. If it is in the radiology or on the floor or on the way to the hospital, I would agree. But if it happens in the operating room, I think one should proceed with the operation. Because we have had success under those circumstances. And since you say that you take these patients to the operating room for both diagnosis and treatment, I would like to suggest that patients with need for cardiopulmonary resuscitation enjoy the magnificent approach you have adopted.
Dr. Joseph E. Bavaria: Thank you very much. Our patients receiving CPR in this series initiated active CPR in the helicopter and through the elevator ride to the operating room. We did operate on these patients, and they all died. But I believe I agree with you. If you have to initiate CPR while you are in the operating room, you should definitely proceed with dissection repair.
Footnotes
Presented at the 121st Annual Meeting of the American Surgical Association on April 26–28, 2001, the Broadmoor Hotel, Colorado Springs, Colorado.
Correspondence: Joseph E. Bavaria, MD, Division of Cardiothoracic Surgery, Hospital of the University of Pennsylvania, 3400 Spruce St., Philadelphia, PA 19104-4283
E-mail: joseph.bavaria@uphs.upenn.edu
Accepted for publication April 26, 2001.
References
- 1.Fuster V, Ip JF. Medical aspects of acute aortic dissection. Semin Thorac Cardiovasc Surg 1991; 3: 219–224. [PubMed] [Google Scholar]
- 2.Hagan PG, Nienaber CA, Isselbacher EM, et al. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA 2000; 283: 897–903. [DOI] [PubMed] [Google Scholar]
- 3.DeBakey ME, Cooley DA, Creech O. Surgical consideration of dissection aneurysm of the aorta. Ann Surg 1955; 142: 586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livesay JJ, Cooley DA, Duncan JM, et al. Open aortic anastomosis: improved results in the treatment of aneurysms of the aortic arch. Circulation 1982; 66 (suppl I): 122–127. [PubMed] [Google Scholar]
- 5.Lemole GM, Strong MD, Spagna PM, et al. Improved results for dissecting aneurysms: intraluminal sutureless prosthesis. J Thorac Cardiovasc Surg 1982; 83: 249–255. [PubMed] [Google Scholar]
- 6.Bavaria JE, Woo YJ, Hall RA, et al. Circulatory management with retrograde cerebral perfusion for acute type A aortic dissection. Circulation 1996; 92 (pt 2):I1793. [PubMed] [Google Scholar]
- 7.Fann JI, Smith JA, Miller DC, et al. Surgical management of aortic dissection during a 30-year period. Circulation 1995; 92 (suppl II): 113–121. [DOI] [PubMed] [Google Scholar]
- 8.David TE, Armstrong S, Ivanov J, et al. Surgery for acute type A aortic dissection. Ann Thorac Surg 1999; 67: 199–2001. [DOI] [PubMed] [Google Scholar]
- 9.Bavaria JE, Woo YJ, Hall RA, et al. Retrograde cerebral and distal aortic perfusion during ascending and thoracoabdominal aortic operations. Ann Thorac Surg 1995; 60: 345–353. [DOI] [PubMed] [Google Scholar]
- 10.Coselli JS, Crawford ES, Beall AC Jr, et al. Determination of brain temperature for safe circulatory arrest during cardiovascular operation. Ann Thorac Surg 1995; 60: 345–353. [DOI] [PubMed] [Google Scholar]
- 11.Stecker MM, Cheung AT, Bavaria JE, et al. Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg 2000; 71 (1): 14–21. [DOI] [PubMed] [Google Scholar]
- 12.Stecker MM, Cheung AT, Bavaria JE, et al. Deep hypothermic circulatory arrest: II. Changes in electroencephalogram and evoked potentials during rewarming. Ann Thorac Surg 2000; 71 (1): 22–28. [DOI] [PubMed] [Google Scholar]
- 13.Fuster V, Ip JF. Medical aspects of acute aortic dissection. Semin Thorac Cardiovasc Surg 1991; 3: 219–224. [PubMed] [Google Scholar]
- 14.Anagnostopoulos CE, Prabhakar MS, Kittle CF, et al. Aortic dissections and dissecting aneurysms. Am J Cardiol 1972; 30: 263–273. [DOI] [PubMed] [Google Scholar]
- 15.DeBakey ME, McCollum CH, Crawford ES, et al. Dissection and dissecting aneurysms of the aorta: twenty-year follow-up of five hundred twenty-seven patients treated surgically. Surgery 1982; 92: 1118–1134. [PubMed] [Google Scholar]
- 16.Morris GC JR, Henley WS, Debakey ME. Correction of acute dissecting aneurysm of aorta with valvular insufficiency. JAMA 1963; 184: 185–186. [Google Scholar]
- 17.Borst HG, Schaudig A, Rudolph W. Arteriovenous fistula of the aortic arch: repair during deep hypothermia and circulatory arrest. J Thorac Cardiovasc Surg 1964; 48: 443–447. [PubMed] [Google Scholar]
- 18.Griepp RB, Stinson EB, Hollingsworth JF, et al. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg 1975; 70: 1051–1063. [PubMed] [Google Scholar]
- 19.Ueda Y, Miki S, Kusuhara K, et al. Surgical treatment of aneurysm or dissection involving the ascending aorta and aortic arch, using circulatory arrest and retrograde cerebral perfusion. J Cardiovasc Surg 1990; 31: 553–558. [PubMed] [Google Scholar]
- 20.Deeb GM, Jenkins E, Bolling SF, et al. Retrograde cerebral perfusion during hypothermic circulatory arrest reduces neurological morbidity. J Thorac Cardiovasc Surg 1995; 109: 738–743. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura M, Hashimoto A, Akimoto T, et al. Operation for type A aortic dissection: introduction of retrograde cerebral perfusion. Ann Thorac Surg 1995; 59: 1195–1199. [DOI] [PubMed] [Google Scholar]
- 22.Svensson LG, Crawford ES, Hess KR, et al. Deep hypothermia with circulatory arrest: determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg 1993; 106: 19–31. [PubMed] [Google Scholar]
- 23.Mills NL, Ochsner JL. Massive air embolism during cardiopulmonary bypass: causes, prevention and management. J Thorac Cardiovasc Surg 1980; 80: 708–717. [PubMed] [Google Scholar]
- 24.Raanani E, Latter D, Erret L, et al. “Bioglue” improves outcome in aortic surgery. Aortic Surgery Symposium Proceedings, 2000: 81. [Google Scholar]
- 25.Kirali K, Rabus MB, Mansuroglu D, et al. Surgical treatment of acute type A aortic dissection: early and long-term results. Aortic Surgery Symposium Proceedings, 2000: 101. [Google Scholar]
- 26.Ghariani S, Liard L, Spaey J, et al. Retrospective study of somatosensory evoked potential monitoring in deep hypothermic circulatory arrest. Ann Thoracic Surg 1999; 67: 1915–1921. [DOI] [PubMed] [Google Scholar]
- 27.Kleine P, Perthel M, Laas J, et al. Mechanisms, monitoring, and management of intraoperative cerebral malperfusion in surgery of type A dissection (AADA). Aortic Surgery Symposium Proceedings, 2000: 91. [Google Scholar]
- 28.Deeb GM, Williams DM, Bolling SF, et al. Surgical delay for acute type A dissection with malperfusion. Ann Thorac Surg 1997; 64: 1669–1677. [DOI] [PubMed] [Google Scholar]
- 29.Takamoto S, Matsuda T, Harada M, et al. Simple hypothermic retrograde cerebral perfusion during aortic arch surgery. J Cardiovasc Surg 1992; 33: 560–567. [PubMed] [Google Scholar]
- 30.Safi HJ, Brien HW, Winter JN, et al. Brain protection via cerebral retrograde perfusion during aortic arch aneurysm repair. Ann Thorac Surg 1993; 56: 270–276. [DOI] [PubMed] [Google Scholar]
- 31.Murase M, Maeda M, Koyama T, et al. Continuous retrograde cerebral perfusion for protection of the brain during aortic arch surgery. Eur J Cardiothorac Surg 1993; 7: 597–600. [DOI] [PubMed] [Google Scholar]
- 32.Lin PJ, Chang CH, Tan PC, et al. Protection of the brain by retrograde cerebral perfusion during circulatory arrest. J Thorac Cardiovasc Surg 1994; 108: 969–974. [PubMed] [Google Scholar]
- 33.Coselli JS, Buket S, Djukanovic B. Aortic arch operation: current treatment and results. Ann Thorac Surg 1995; 59: 19–27. [DOI] [PubMed] [Google Scholar]
- 34.Pagano D, Carey JA, Patel RL, et al. Retrograde cerebral perfusion: clinical experience in emergency and elective aortic operations. Ann Thorac Surg 1995; 59: 393–397. [DOI] [PubMed] [Google Scholar]
- 35.Lytle BW, McCarthy PM, Meaney KM, et al. Systemic hypothermia and circulatory arrest combined with arterial perfusion of the superior vena cava. J Thorac Cardiovasc Surg 1995; 109: 738–743. [DOI] [PubMed] [Google Scholar]