Abstract
Objective
To assess the use of surgical procedures by tumor location and compliance with adjuvant therapy recommendations by tumor stage. The study was conducted in a population-based setting to identify target patient groups for improved care.
Summary Background Data
Rectal cancer therapy potentially involves similar patients receiving different treatments. Low anterior resection (LAR), sparing the anal sphincter, and abdominoperineal resection (APR), ablating the anal sphincter, offer equivalent local recurrence and survival rates but may differ in quality of life measurements. The 1990 NIH Consensus Conference recommended that patients with stage II and III rectal cancer receive radiation and chemotherapy in conjunction with surgical resection, but this is not uniformly applied. To interpret the use of these therapies, information on tumor location in the rectum, which is rarely known in population-based studies, is necessary. Patient, hospital, or surgeon characteristics may influence which procedure is performed and whether adjuvant therapy is given.
Methods
Information about primary, invasive rectal adenocarcinomas diagnosed between 1994 to 1996 in 13 California counties was obtained from the regional cancer registry. Tumor location, determined from abstracted medical text, was divided into the upper, middle, and lower rectum. Hospitals were characterized by teaching status, number of beds, and cancer center designation. Surgeons were categorized as general or colorectal surgeons. Factors associated with a higher use of LAR versus APR in patients with middle and lower rectum tumors and factors associated with a higher use of NIH-recommended therapy in patients with stage II and III disease were separately analyzed.
Results
Among 637 eligible patients, APR was used in 22% of those with middle rectum tumors and 55% of those with lower rectum tumors. Factors significantly associated with a higher use of LAR included female gender, middle rectum location, and treatment in a major teaching hospital versus a nonteaching hospital. Recommended therapy was received by 44% of patients with stage II disease and 60% of those with stage III disease. Factors significantly associated with higher compliance with NIH recommendations included age younger than 60 versus older than 75, age 60 to 75 years versus older than 75, tumor location in the middle or lower rectum versus the upper rectum, stage III disease, and treatment at a teaching hospital versus a nonteaching hospital.
Conclusions
Patients with similar rectal cancers receive different treatments independent of tumor stage or location. This may result in more APRs performed for middle and lower rectum tumors than necessary and less adequate treatment for stage II and III tumors than recommended.
Cancer of the rectum newly afflicts 36,000 people and causes 8,800 deaths each year in the United States. 1 Based on investigational studies, surgical therapy for rectal cancer underwent considerable changes during the latter half of the 20th century. In 1940 more than 80% of patients with rectal cancer were treated with an abdominoperineal resection (APR), a procedure that involves removal of the entire rectum and anus, resulting in a permanent colostomy; now APR is performed in only 20% to 30% of patients. 2 Data from these studies showed no significant difference in overall patient survival rates or local recurrence rates when similar tumors were resected with an APR or a low anterior resection (LAR), a less radical procedure that removes only a portion of the rectum, allowing restoration of bowel continuity. 3–9 In addition, large randomized trials have shown that adjuvant radiation therapy and chemotherapy decrease local recurrence rates and increase disease-free survival rates for many rectal cancers. 10–16 The strength of such evidence resulted in a 1990 National Institutes of Health (NIH) Consensus Development Conference statement concluding that patients with stage II and III rectal cancer, defined as invading beyond the submucosa or involving regional lymph nodes, should be treated with a combination of surgical resection, chemotherapy, and radiation. 17
Despite these developments, complication and death rates from rectal cancer remain high. One possible explanation lies in the variable application of available therapies. Variation in therapy use has already been shown in rectal cancer, among many other diseases. 18–25 Surgeons with different types of training and institutions with varying volumes of patients with cancer provide rectal cancer care. We hypothesized that similar patients with similar tumors would receive different treatment depending on where and from whom they seek treatment, and some of these variations in treatment potentially represented suboptimal patient care.
Prior studies have attempted to identify the nature of therapy variations, but these have several general limitations. Large population-based studies (i.e., the National Cancer Data Base reports from the American College of Surgeons Committee on Cancer and the American Cancer Society) show the variation in rectal cancer therapy over time but often lack the clinical information needed to interpret these variations. For example, one of the most important clinical elements in understanding surgical rectal cancer therapy is tumor location within the rectum, information that is rarely available in population-based studies. However, institution-specific studies, which do often contain more clinical information, are not reflective of practices in a general population. Ideally, a study examining both general therapeutic practices and sources of practice variation would combine the clinical information needed to interpret variation with a population-based setting, thus allowing evaluation of appropriate therapy delivery to all relevant members of a population.
The purpose of this study was to assess the use of surgical procedures and adjuvant therapy in the initial treatment of patients with rectal cancer and to identify patient, hospital, and surgeon characteristics associated with certain variations in treatment patterns. Two specific patient groups were separately examined for treatment variation: patients with middle and lower rectal tumors for greater use of LAR versus APR, and patients with stage II and III disease for greater compliance with recommended therapy as outlined by the 1990 NIH Consensus Conference.
METHODS
Case Identification
The study design was a cross-sectional analysis of all incident cases of rectal cancer collected by the Cancer Surveillance Program, Region 3, of the California Cancer Registry during a 3-year period. The area encompassed 13 urban and rural counties around Sacramento. The area’s population was estimated at 2.8 million people in 1995. 26 The annual age-adjusted incidence of cancer in the rectum and rectosigmoid in this region between 1992 and 1996 was 15.4 per 100,000 population for men and 9.3 per 100,000 population for women. 26 Cases were documented in the registry by patient residence, even if medical care was sought outside the region. California state law mandates case reporting, and cases are reported to the Cancer Surveillance Program by hospitals approximately 6 months after diagnosis, allowing time for completion of initial therapy. The California Cancer Registry estimated more than 98% complete ascertainment of nonprostate cancer cases for 1996. 27
All patients with rectal cancer diagnosed between January 1 1994, and December 31 1996, and reported to the Cancer Surveillance Program before October 1998 were identified. Exclusion criteria included recurrent rectal cancer, carcinoma in situ, primary location coded to rectosigmoid or anus, and histology not consistent with adenocarcinoma. Stage at diagnosis for all patients was assigned in accordance with American Joint Committee on Cancer (AJCC) criteria. 28 Because of the presence of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program in California, all cancer registry cases are staged in accordance with the SEER-stipulated Extent of Disease criteria. Hospitals designated as American College of Surgeons-approved cancer centers additionally report AJCC staging. Cases for which AJCC staging was not originally reported were converted to AJCC staging with a computer software application provided to the registry by SEER. 29 The regional registry conducted a study evaluating this application’s accuracy and found approximately 90% concordance with cancer registrar staging. 30
In addition to stage at diagnosis, patient age, gender, and race were known for each case. Information involving personal or family medical history is not collected by the Cancer Surveillance Program. Treatment variables included type of surgical procedure, reason for no surgery, receipt of radiation therapy, sequence of radiation relative to surgery, and receipt of chemotherapy. Surgical procedure was coded in one of the following categories: local excision, local destruction, LAR or other segmental proctectomy, total proctectomy (APR), proctectomy with en bloc resection of other organs, and surgery not otherwise specified. 31 Several procedures were combined into an “other procedures” category because they either were not performed for curative intent (e.g., local destruction) or were not uniform in the type of procedures described (e.g., surgery not otherwise specified or proctectomy with resection of other organs). All coded therapy was considered part of the first comprehensive treatment course directed at this specific cancer. Dichotomous variables for receiving chemotherapy or radiotherapy were used because detailed information on either treatment regimen was not available. Information on chemotherapy may be missing in some patients because this may be administered solely in an outpatient setting.
Tumor Location
Beginning with all patients reported in November 1994, abstractions from patient admission, surgery, radiology, endoscopy, and pathology reports found in the medical chart were stored electronically as text fields by the state registry. Their purpose was to document all diagnostic findings that describe the tumor as fully as possible. To assess the abstractions’ utility in providing specific tumor location information, a random set of text fields was sampled before conducting the study. This data showed that tumor location could be confidently assigned in 86% of patients. The primary author reviewed the reports for each patient in the study. Tumor location assignment was made only if one of the following was present within the text field entries: a measured distance to the tumor from a defined reference point; a direct reference to the upper, middle, or lower rectum; or an unambiguous description of anatomic location. Examples of the latter included “tumor involving the sphincter” and “directly inside the anal verge.” Tumor location was not assigned if the available descriptions did not clearly indicate location. Tumor location identification was most often derived from surgery or endoscopy notes. When reports provided conflicting information, priority was given to sources in the following descending order: intraoperative observations, pathology specimen, rigid endoscopy, flexible endoscopy, physical examination, and radiologic procedures. This ranking was used to reflect known discrepancies between the tools used to describe rectal tumor location. The anal verge was selected as the standard reference point for all measurements of tumor distance from the anus. 32 One additional centimeter was added to all measurements identifying other reference points (e.g., dentate line) to standardize tumor location. To lessen the effect of measurement inaccuracies, location designations were divided into the following categories: lower rectum (0–5 cm), middle rectum (6–10 cm), and upper rectum (11–15 cm from the anal verge).
Physician and Hospital Characteristics
The registry linked hospital and surgeon identifiers with specific characteristics for each patient in accordance with registry confidentiality policy. After confirmation of the primary surgeon affiliated with each patient, where possible, the registry coded the self-reported specialty of each surgeon from American Medical Association physician information, which is recorded regardless of association membership. 33 Specialty designations reflected only specialties with both relevance to rectal cancer therapy and separate board certifications. Therefore, general surgery and colorectal surgery were the two included specialties. Hospital characteristics were coded in four categories: number of beds, teaching status, location inside/outside Region 3, and designation as an American College of Surgeons-approved cancer program. Number of beds was calculated as an average for the 3 years of study and then categorized into three groups: 100 beds or less, 101 to 299 beds, and 300 beds or more. 34,35 Teaching status was designated by the following four categories:
Members of the Council of Teaching Hospitals: Membership is granted by the Association of American Medical Colleges to hospitals affiliated with an approved medical school and sponsoring a minimum of four residency programs, at least two of which must be in medicine, surgery, obstetrics/gynecology, pediatrics, psychiatry, or family practice. This designation includes most major academic medical centers.
Major teaching hospitals: This category includes non-Council of Teaching Hospital members that sponsor at least four residency programs, two of which are internal medicine and surgery.
Minor teaching hospitals: These institutions sponsor or participate significantly in fewer than four residencies and do not participate in both an internal medicine and general surgery residency.
Nonteaching hospitals: These hospitals have no affiliation with a medical school or residency program. 34–37
Location of primary treatment at a facility inside or outside Region 3 was noted. Designation as an American College of Surgeons-approved cancer center was dichotomous, with no differentiation between types of approval status. 34,35
Statistical Analysis
The use of surgical procedures and adjuvant therapy was evaluated with patient, surgeon, and hospital characteristics using the Pearson chi-square test for categorical variables and the Student t test or analysis of variance for continuous variables. Separate analyses were performed with relevant subsets of the data to examine associations with higher use of desirable treatment modalities. Thus, patients with middle and lower rectum tumors were assessed for greater use of LAR rather than APR, and patients with stage II and III tumors were assessed for greater use of NIH-recommended therapy. To verify the significance of associations identified in bivariate analysis, logistic regression models were used to adjust for related factors. Again, separate models with reduced data subsets were used. Odds ratios for undergoing a LAR rather than an APR were calculated, evaluating only patients with tumors of the middle or lower rectum treated with LAR or APR (n = 237). Similarly, odds ratios for receiving NIH-recommended therapy were derived including only stage II and III patients in the model (n = 291). Within the recommended therapy model, inclusion of tumor location as an independent variable further reduced the available number of observations (n = 194). Both the full and reduced models were evaluated, and no appreciable change in the estimates for the other included variables was detected between the two models. Therefore, the reported odds ratios and confidence intervals reflect the full model, except for odds ratios regarding tumor location. Because of multiple testing performed for certain outcomes, values of P > 0.01 were interpreted more cautiously. Analytic tests were performed with Stata 5.0 software (STATA Corp., College Station, TX).
RESULTS
Characteristics
During the 3-year study, 740 patients with primary, invasive rectal cancer were reported among the Region 3 population. Those excluded based on pathology consisted of 34 carcinoid tumors, 3 malignant lymphomas, and 4 squamous cell carcinomas. Twenty-five patients staged by the computer conversion program as having in situ carcinoma by AJCC criteria were also excluded. Finally, 37 patients could not be definitively staged despite manual review of the charts at the registry and were excluded. A total of 637 patients remained for analysis. A set of descriptive text fields was available for 497 patients. Tumor location could be determined from these text fields in 407 patients (82%). A total of 65 hospitals were represented as sites of primary treatment for the study patients. Table 1 describes the study patients by patient, tumor, hospital, and surgeon characteristics.
Table 1. GENERAL CHARACTERISTICS OF INVASIVE RECTAL CANCER CASES, CANCER SURVEILLANCE PROGRAM, REGION 3 OF THE CALIFORNIA CANCER REGISTRY, 1994–1996
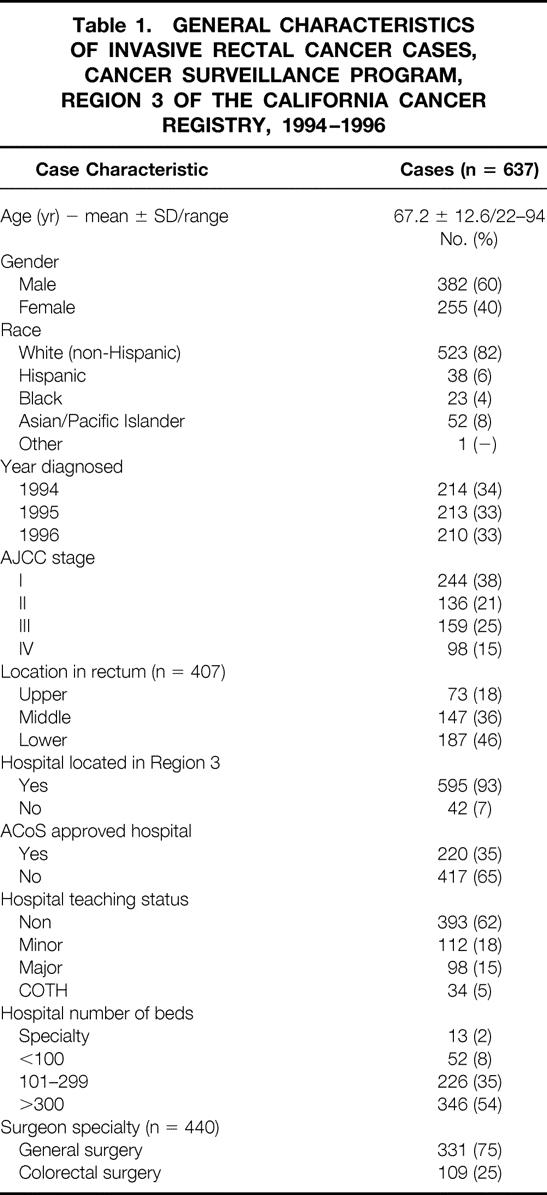
Tumor stage and location followed distribution patterns reported elsewhere in that tumors appeared to be more prevalent in the lower and middle rectum. 24,38 Importantly, no difference in distribution of disease stage was found among the three areas of the rectum. The identity of the operating surgeon was available in 440 instances (69%). A general surgeon treated three quarters of the patients for whom a surgeon was identifiable. Table 2 describes the patient characteristics associated with surgeons by specialty. Average patient age was highest among those on whom a general surgeon operated. Although tumor stage distribution was equivalent by surgeon specialty, colorectal surgeons treated more tumors located in the lower rectum compared with general surgeons. Lastly, patients on whom colorectal surgeons operated were affiliated with teaching hospitals and large hospitals in a greater proportion than patients on whom general surgeons operated.
Table 2. DESCRIPTIVE CHARACTERISTICS OF CASES BY SURGEON SPECIALTY
* Reduced numbers of observations: GS = 218; CRS = 84; Unk = 105.
Current Use of Surgical Procedures and Adjuvant Therapy
The types of surgical procedures performed and adjuvant therapy received for the initial treatment of rectal cancer are shown in Table 3. The results are stratified by stage and location of disease. A surgical intervention was part of treatment for 575 (90%) patients. With the exception of local excision in stage I disease and no surgery in stage IV disease, LAR and APR made up the predominant surgical treatments. Even in stage I disease, LAR and APR combined accounted for nearly 60% of resections. Use of the procedures in patients with stage II and III disease was essentially equal.
Table 3. PROPORTION OF CASES TREATED SURGICALLY AND WITH RADIOTHERAPY OR CHEMOTHERAPY, STRATIFIED BY STAGE AND LOCATION OF DISEASE
Surgical procedure category ‘other’ includes local destruction, proctectomy plus en bloc resection of another organ, and surgery not otherwise specified. Patients could receive radiation and chemotherapy alone or in combination; therefore percentages in the adjuvant therapy column do not equal 100%.
Unlike stage, tumor location had a considerable impact on the type of procedure performed. The proportion of patients undergoing local excision increased with greater proximity of the tumor to the anal sphincter, as did proportional use of APR. In the upper rectum, where resection should be technically feasible without compromising the anal sphincter, no tumors were removed with an APR. In the middle rectum, where theoretically the most potential for reducing the number of sphincter-ablating procedures exists, 22% of tumors overall were removed by APR. More than half of lower rectal tumors were resected with an APR.
Colorectal surgeons performed fewer APRs for middle rectal tumors than general surgeons. Among operations performed by colorectal surgeons, resection of middle rectal tumors occurred by LAR in 57%, APR in 13%, local excision in 17%, and another procedure in 13%. In contrast, patients treated by general surgeons underwent LAR in 57%, APR in 29%, local excision in 9%, and another procedure in 5%. However, the differences between specialties did not achieve statistical significance in this sample of 108 middle rectal tumors with known surgeon specialty (P = .18).
More than 93% of patients with stages I, II, or III disease underwent surgical resection. Of the 62 patients of all stages who did not undergo surgery, there was no physician recommendation for surgery in 46 (74%). More than 75% of patients without a physician recommendation were classified as having stage IV disease. Other reasons for not having surgery included patient or family refusal (15%) and the presence of a medical contraindication (3%). In four patients (6%), a physician recommendation for surgery was present in the medical record, but it was unknown whether the patient ultimately received surgical treatment elsewhere.
Table 3 also shows the proportion of patients receiving radiation therapy or chemotherapy by stage and location of tumor. Slightly more than 50% of patients with stage II tumors received radiation or chemotherapy; these therapies were applied in greater proportion to patients with stage III disease. Differential application of adjuvant therapy was also evident by tumor location. Therapy use increased for the middle and lower rectum tumors compared with the upper rectum, despite equal distributions of disease stage for all three areas of the rectum. Of the 236 patients who underwent radiotherapy within this sample, 72% received radiation after surgery, 17% before surgery, and only 1% both before and after surgery. The sequence of therapy was unknown for the remaining 10% of patients receiving radiation.
Therapy combinations for individual patients with stage II and III disease were examined to assess accordance with NIH Consensus Conference recommendations. Patients with stage III disease received recommended therapy in significantly higher proportions than those with stage II disease (P < .01). Of 136 patients with stage II disease, 44% received a combination of surgery, radiation, and chemotherapy in accordance with NIH recommendations. In 159 patients with stage III disease, 60% underwent combined surgery, radiation, and chemotherapy. As a comparison, surgery without any adjuvant therapy was reported in 38% of stage II patients and 28% of stage III patients.
Factors Associated With Higher Use of Low Anterior Resection in Middle and Lower Rectum
Comparisons between patients treated with LAR or APR for middle or lower rectal cancer showed no significant differences between the two groups in mean patient age, race, tumor stage distribution, surgeon specialty, or hospital characteristics. Female gender was associated with higher use of LAR in that 52% of the women with middle or lower rectum tumors underwent LAR versus 38% of the men (P = .04). As expected, tumor location bore the strongest correlation with type of surgical resection: LAR was used in 71% of middle rectum tumors and 19% of lower rectum tumors (P < .001).
Tumors treated by general surgeons and colorectal surgeons were resected with LAR in 42% and 43% of middle and lower rectal tumors, respectively. To evaluate whether a practice difference existed between the specialty groups for performing sphincter-sparing procedures versus sphincter-ablating procedures, analysis was repeated defining sphincter-sparing procedures as LAR or local excision and sphincter-ablating procedures as APR. Again, no significant difference emerged, with 46% of patients operated on by a general surgeon undergoing a sphincter-sparing procedure compared with 51% of patients operated on by a colorectal surgeon among middle and lower rectal tumors (P = .64). In middle rectal tumors, 66% of patients operated on by a general surgeon and 74% of those operated on by a colorectal surgeon underwent a sphincter-sparing procedure (P = .14). In lower rectal tumors, 26% of patients operated on by a general surgeon and 39% of those operated on by a colorectal surgeon underwent a sphincter-sparing procedure (P = .34).
Finally, a greater proportion of patients at teaching hospitals and at large hospitals underwent a LAR. At nonteaching hospitals, 38% of patients with middle and lower rectal tumors were treated with LAR compared with 49% at minor teaching hospitals, 59% at major teaching hospitals, and 44% at Council of Teaching Hospitals members (P = .11). At hospitals smaller than 100 beds, 33% of patients underwent LAR, contrasted with 38% at hospitals with 101 to 299 beds and 50% at hospitals larger than 300 beds (P = .15).
Adjusted odds ratios (OR) relating these factors with the use of LAR over APR in patients with middle or lower rectal tumors are presented in Table 4 to verify the validity of these associations. The odds that a woman with a middle or lower rectal tumor underwent LAR rather than APR were twice that of a man (OR = 1.99) after adjusting for patient age, tumor stage, hospital characteristics, and surgeon specialty. Stage of disease did not substantially influence use of LAR over APR. Tumor location functioned as the most influential determinant of procedure type: middle rectal tumors were 19 times more likely to be resected with LAR than lower rectal tumors. For the patients with both known surgeon specialty and tumor location and thus available for this analysis, surgeon specialty did not significantly affect the type of procedure performed. Lastly, the only hospital characteristic that appreciably affected higher use of LAR was teaching status. Patients treated with LAR rather than APR were more than five times more likely to be treated at a major teaching hospital (OR = 5.19) than at a nonteaching hospital (OR = 1.0) or a minor teaching hospital (OR = 1.03). This finding, however, did not extend to patients treated at Council of Teaching Hospitals members (OR = 0.43).
Table 4. ODD RATIOS FOR RECEIVING A LAR RATHER THAN AN APR FOR MIDDLE AND LOWER RECTAL TUMORS
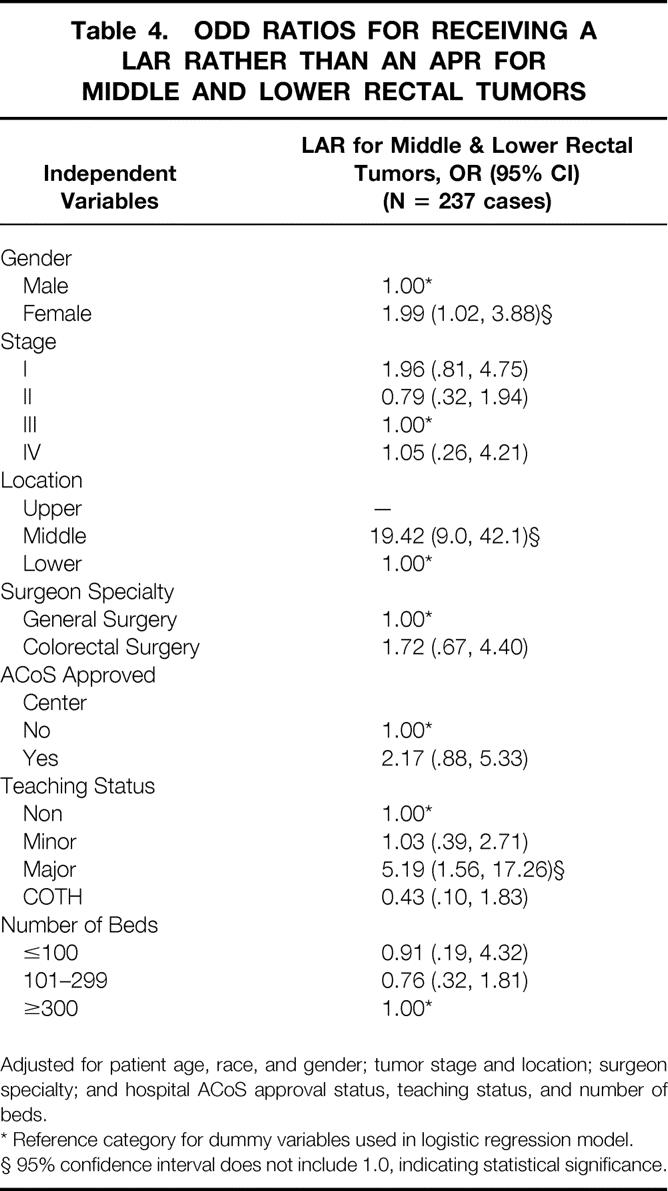
Adjusted for patient age, race, and gender; tumor stage and location; surgeon specialty; and hospital ACoS approval status, teaching status, and number of beds.
* Reference category for dummy variables used in logistic regression model.
§ 95% confidence interval does not include 1.0, indicating statistical significance.
Factors Associated With Higher Use of Recommended Therapy in Stages II and III
A similar but separate evaluation was performed for the higher use of NIH-recommended therapy in treating stage II and III rectal tumors. The most remarkable association was that of increasing age with decreasing compliance with recommended therapy. For patients younger than age 59, 73% received recommended therapy, in contrast to only 25% of patients older than age 76. Therefore, even among the youngest patients, one in four did not receive recommended therapy. Patient gender did not appear to influence compliance: 56% of men and 48% of women received NIH-recommended therapy (P = .15). Patient race was not significantly associated with a higher use of recommended therapy. As noted previously, an increased accordance with recommended therapy in patients with stage III disease (60%) compared with stage II disease (44%) was evident (P < .01). Patients with stage II and III tumors in the upper rectum were treated with recommended therapy 36% of the time, compared with 55% of those with middle rectal tumors and 59% of those with lower rectal tumors (P = .09).
The NIH-recommended therapy was given to 67% of patients treated in minor teaching hospitals and 63% of patients treated in major teaching hospitals, compared with 48% of patients treated in nonteaching hospitals and 36% of patients treated in Council of Teaching Hospitals members (P = .03). Average patient age, tumor stage distribution, or tumor location distribution, none of which differed significantly between institutions grouped by teaching status, did not explain these differences in compliance. Although not statistically significant, greater proportions of patients received recommended therapy at larger hospitals and at American College of Surgeons-approved cancer centers.
Table 5 lists the OR for compliance with NIH-recommended therapy after adjustment for other independent variables. Patients receiving recommended therapy were four times more likely to be age 60 to 75 (OR = 4.2) compared with older than 76, using the older-than-age-76 patients as the reference group. The adjusted OR of getting recommended therapy was nearly 10 times greater for patients younger than age 60 (OR = 9.52) than that of patients older than age 76. Use of recommended therapy was 2.5 times more likely in patients with stage III tumors compared with those with stage II tumors. Application of NIH treatment recommendations was more than three times as likely in patients with middle (OR = 3.34) or lower (OR = 3.22) rectal tumors than in patients with upper rectal tumors. Lastly, patients receiving recommended therapy were twice as likely to be treated at minor teaching hospitals (OR = 2.60) or major teaching hospital (OR = 2.35) than at nonteaching hospitals. The difference between major teaching hospitals and nonteaching hospitals did not quite reach statistical significance, but major and minor teaching hospitals appeared similar in their treatment records. In contrast, Council on Teaching Hospital members displayed a lower use of recommended therapy and did not mirror the practice pattern seen at other teaching hospitals. The number of cases treated at Council of Teaching Hospitals institutions was too small in this sample to allow definitive conclusions to be reached; nevertheless, the differences from other teaching institutions could not be readily explained by the information available in this study.
Table 5. ODDS RATIOS FOR RECEIVING COMBINATION SURGERY, RADIATION, AND CHEMOTHERAPY FOR STAGE II AND III RECTAL TUMORS

Adjusted for patient age, race, and gender; tumor stage and location; surgeon specialty; and hospital ACoS approval status, teaching status, and number of beds.
* Reference category for dummy variables used in logistic regression model.
§ 95% confidence interval does not include 1.0, indicating statistical significance.
DISCUSSION
This description of surgical and adjuvant rectal cancer therapy confirms that patients with similar tumors may receive different procedures or treatment modalities independent of tumor location or stage. This study identifies certain patient groups that may have decreased access to optimal therapy, warranting a reevaluation of practice patterns. Finally, this research highlights the strengths and limitations of cancer registry data and helps to identify which information is most important in interpreting rectal cancer care within a population.
Interpreting the use of surgical procedures for this population is difficult because the appropriateness of the individual procedures cannot be determined. Arguably, all middle rectum tumors and some lower rectum tumors can be resected with either APR or LAR. The ultimate choice of procedure, therefore, rests with the individual surgeon, and the considerations in this decision-making process are not known. These include, among others, the technical feasibility of obtaining adequate resection margins and of reconstituting bowel continuity, the patient’s overall health status, and the patient’s preoperative anal sphincter function. Historical comparisons thus serve as the closest standard against which to judge the use of surgical procedures within a population. The American College of Surgeons Committee on Cancer case reviews showed that 29% of the patients with rectal cancer seen at 943 U.S. centers in 1983 underwent APR, as did 25% of the patients in 1988. These statistics, however, included patients with in situ and unknown-stage disease, which may lower the proportions of APR. A concomitant 5% increase in the number of LAR procedures was seen. 25 Our study indicates that 31% of patients with stages I through IV disease underwent APR, suggesting that there has been no significant change recently in the overall application of APR. The large population-based studies reporting patients from the 1980s and early 1990s did not indicate therapy by tumor location, information needed to gauge the use of APR among middle rectal tumors. To obtain this information, institutional series comparing recurrence and survival rates in patients after APR or LAR, which sometimes reflect surgical therapy by tumor location, must be sought. Thirty-eight percent of tumors located within 5 to 7 cm of the anal verge and occurring between 1980 and 1991 were resected with APR, according to a Cleveland Clinic study. 39 In two European reports, 40% to 50% of middle rectum tumors were resected with APR. 40,41 This contrasts with 22% of patients with middle rectal tumors who underwent APR in our study.
Examining surgical procedures by stage and location shows potential areas for further reduction in the use of more radical procedures. For instance, nearly 60% of patients with stage 1 tumors in this study underwent either APR or LAR, implying use of relatively radical procedures for early-stage disease. Improved preoperative staging through endorectal ultrasound may decrease the use of these procedures. Increased future use of preoperative radiation may reduce the need for APR to resect middle rectum tumors. Within this population, only 17% of irradiated patients were known to receive this therapy before surgery.
Both historical comparisons and guidelines such as the NIH Consensus Conference statement aid the interpretation of adjuvant therapy use. A dramatic increase in the use of adjuvant therapy was evident in this study. The combination of surgery, radiation, and chemotherapy was used in 20% of patients with stage II disease and 35% of those with stage III disease from U.S. treatment centers reporting to the National Cancer Data Base in 1990. 24 The current study showed that NIH-recommended therapy was given in 44% of patients with stage II disease and 60% of those with stage III disease. This suggests a diffusion of knowledge and adoption of practice since the publication of the 1990 NIH Consensus Conference report.
Despite these improvements, the administration of adjuvant therapy for patients with stage II and III disease, as recommended by these guidelines, presumably still falls short of desired levels. This consensus conference did not state specific contraindications to its recommendations. Clearly, not every patient with stage II or III rectal cancer is a candidate for or agreeable to all three therapies. Because this patient information is not known, it is impossible to estimate an ideal compliance rate for this population. Nevertheless, because there are no clinically justifiable reasons to treat stage II patients less aggressively than stage III patients, the stage II compliance rate should be at least that of stage III. There were no differences in patient age, hospital characteristics, or tumor location between stage II and III patients in this study to explain discrepancies in the compliance rate. Clinicians need to emphasize increasing therapy compliance among patients with stage II rectal cancer as an opportunity to reduce the death and complication rates of this disease.
The disparity in compliance rates by tumor location provides another opportunity to reevaluate rectal cancer treatment. This study showed that patients with stage II and III tumors in the middle or lower rectum were significantly more likely to receive NIH-recommended therapy than those with tumors in the upper rectum. To test whether this finding reflects data suggesting that upper rectum tumors respond more like sigmoid colon lesions rather than rectal cancers and therefore do not warrant radiation therapy, a different criterion of appropriate care was defined and applied to these patients. These criteria would accept, for patients with stage III upper rectal tumors, either NIH-recommended therapy or surgery plus chemotherapy and, for patients with stage II upper rectal tumors, either NIH-recommended therapy, surgery alone, or surgery plus chemotherapy. The NIH-recommended therapy would continue to serve as appropriate care for patients with middle and lower rectal tumors. Applying this new standard, the disparity in compliance between patients with stage II and III tumors was narrowed: now 51% of patients with stage II disease and 62% of those with stage III disease would be considered in compliance with this standard. The previously noted difference in compliance by tumor location was no longer present. Compliance with this broadened standard was found in 59% of patients with lower, 55% of middle, and 64% of upper stage II and III rectal tumors. However, among the 19 patients with known stage III disease in the upper rectum, 42% underwent surgery alone and would not be considered as having appropriate therapy by either definition.
By delineating dissimilarities in treatment, this study focuses attention on certain patient groups who may not be receiving optimal therapy. The stark difference in receipt of adjuvant therapy between younger and older patients certainly warrants continued investigation. The degree to which medical contraindications or patient refusal justifies this difference should be relatively easy to document and quantify, but the information is rarely available in larger studies. Further, investigation into the influence on medical decision-making of biased perceptions of the elderly not being able to tolerate or not wishing full treatment is needed. Studies specifically addressing cancer treatment outcomes in the elderly would also greatly help to interpret the appropriateness of care for this patient group.
The disparate use of APR by gender, although not a substantial difference, may offer an opportunity to reduce the use of this procedure. The Commission on Cancer report also documented the higher use of APR in men than in women. 25 One can speculate that this is a result of the greater technical ease in reconstituting bowel continuity in women because of the anatomic considerations of the pelvis. It has been anecdotally suggested that biased perceptions toward the acceptability of a colostomy by either gender may also play a minor role in this decision. To interpret rates of APR versus LAR, more information on the factors influencing surgeons’ decisions will need to be collected. Perhaps more importantly, a better understanding of which patient-centered outcomes are truly significant in rectal cancer care is merited.
The treatment site also influenced the type of procedure and receipt of adjuvant therapy in this study. A relatively strong association was found between the greater use of LAR in patients with middle and lower rectal tumors who were treated at major teaching institutions. A lesser association was found between higher use of combination therapy among patients treated at minor and major teaching hospitals. Similarly, larger studies such as that from the Commission on Cancer reported a greater use of sphincter-sparing procedures at hospitals with higher cancer caseloads but did not find a significant variation in multimodality therapy by hospital characteristics. 25 Reasons for our findings include the slightly younger mean patient age at teaching hospitals, as well as the presumed likelihood of higher cancer case volumes and concentration of specialists. In a study of 683 patients undergoing LAR or APR at five general hospitals in Edmonton, Canada, between 1983 and 1990, 44% of patients underwent resection with APR. The study did not describe the procedure based on tumor location; however, it did show improved outcomes in patients who were operated on by both colorectal surgeons and surgeons performing more than 21 resections, the median number of resections performed by the surgeons during the study period. The study also showed that colorectal surgeons treated more patients with lower and middle rectal tumors but performed fewer APRs. 23 Although the finding that colorectal surgeons treated a larger proportion of patients with middle and lower rectal tumors with sphincter-sparing procedures than general surgeons was also seen in our study, the difference did not achieve statistical significance. However, surgeon specialty and tumor location were the two variables with the most missing data points in this study. Power in a comparison involving both these variables simultaneously was reduced and was probably the reason that a clear difference between the two specialty groups could not be established or refuted in this study.
Finally, our study shows the advantages and limitations of cancer registry data. The study illustrates the clear importance of tumor location information in interpreting the use of rectal cancer therapy. This study marks the first time these electronic text fields, from which tumor location was extracted, were used for research purposes. Although tumor location was not known for all patients, these texts proved useful overall in providing information not commonly available in population-based data sources. All missing text fields belonged to patients from 1994, because this new information was not required before November 1994. Therefore, only 35% of patients diagnosed in 1994 had text fields present, and tumor location was indicated in 73% of these patients. In contrast, 100% of patients diagnosed in 1995 and 1996 had text fields available, and tumor location was discernible in 83% of these patients. All 1994 patients remained in the analysis because no difference in patient or tumor characteristics was present between 1994 patients with descriptive text fields and patients without text fields.
We did not seek to examine ultimate treatment outcomes (e.g., local recurrence or disease-free survival) because a primary goal was to reflect relatively current practices both within this population and across a broad spectrum of treatment centers. Region 3 encompassed 38 hospitals. In addition, 29 institutions outside Region 3 were represented in this study. Only 42 patients (7%) received primary treatment outside Region 3 facilities. The institutions described in this study truly represented the sites of primary treatment. For instance, only 8 of 575 patients receiving surgical treatment for rectal cancer were reported to the Cancer Surveillance Program by a hospital other than the institution performing the procedure. In those patients, the actual operating institution was not identified. The generalizability to current practice is further strengthened in that case reporting is mandatory. To our knowledge, this is the first rectal cancer therapy study to reflect surgical practice by tumor location within a population-based setting.
As in previous studies, a significant limitation of this research involves missing clinical information that influences medical decision making. Although a documented tumor location was known, certain patient characteristics and intraoperative findings that influence the choice of surgical procedure were not available. More thorough information on physician characteristics, reasons a treatment was not given, and patient payment status could feasibly be collected and would enhance this data source.
An additional limitation results from registry confidentiality policies toward individual institutions and physicians. Basic characteristics of the treating hospital and surgeon could be attributed to most patients, but case volume or case mix of individual hospitals or physicians could not be defined. This limits the comparisons that can be made between, and the conclusions that can be drawn about, particular characteristics of hospitals or surgeons. More accurate collection of physician identifier information by the registry would be important in enhancing its utility. In particular for investigating cancer treatments, it would be helpful if surgical oncology training could be recognized among surgeons, but no reliable means for doing this was identified for this study.
A final limitation is the potential for inaccuracies or underreporting inherent in all registries. The preference for using pathologic rather than clinical staging may result in understaging of patients receiving preoperative radiation. This was unlikely, however, to pose a substantial problem in this study because of the small number of patients irradiated before surgery. Among stage 1 patients who underwent APR or LAR, for example, only 6% had preoperative radiation. The practice of reporting patients within 6 months of diagnosis may cause some underreporting if adjuvant therapy is given long after surgery or if therapy is administered entirely outside a hospital setting, as occasionally occurs with chemotherapy.
Despite the potential flaws of cancer registry data, one of the more positive, far-reaching conclusions derived from this study is that cancer registries can be very valuable in compiling thorough information about populations with an emphasis on clinical detail not found in most administrative databases. In recent years, cancer registries have expanded their role beyond cataloging patients with cancer for epidemiologic purposes to becoming repositories of clinical, treatment, and outcome data. Registries will serve a broader role in evaluating the care of patients with cancer as more detailed treatment and outcome information is collected. Ideally, we can improve the care and survival of current patients with cancer by using such data to investigate which patients are not receiving optimal therapy and why such variations in practice exist.
Acknowledgments
The authors appreciate the efforts of the Cancer Surveillance Program, Region 3, of the California Cancer Registry, particularly Scott Riddle for preparing this dataset and Kathleen Davidson-Allen for her support.
Footnotes
Correspondence: Anneke Schroen, MD, MPH, Department of Surgery, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75235-9159.
E-mail: aschro@meduct.swmed.edu
Supported by the Robert Wood Johnson Clinical Scholars Program.
Cancer incidence data used in this article were collected by the Cancer Surveillance Program, Region 3 under Subcontract 050 mol/L-8703-S1524 with the Public Health Institute. The subcontract is supported by the California Department of Health Services as part of its statewide cancer reporting program, mandated by Health and Safety Code Section 103875 and 103885. The ideas and opinions expressed herein are those of the author, and no endorsement of the State of California, Department of Health Services, or the Public Health Institute is intended or should be inferred.
Accepted for publication January 29, 2001.
References
- 1.National Cancer Institute Cancer Statistics. Cancer Cancer J Clin 1998; 48:6–29.
- 2.Kodner IJ, Fry RD, Fleshman JW, et al, eds. Colon, rectum, and anus, 6th ed. Principles of Surgery, Vol. 2. New York: McGraw-Hill; 1994:1191–1306.
- 3.Stearns MJ. The choice among anterior resection, the pull-through, and abdominoperineal resection of the rectum. Cancer 1974; 34: 969–971. [DOI] [PubMed] [Google Scholar]
- 4.Wolmark N, Fisher B. An analysis of survival and treatment failure following abdominoperineal and sphincter-saving resection in Dukes B and C rectal carcinoma. Ann Surg 1986; 204: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams NS, Durdey P, Johnston D. The outcome following sphincter-saving resection and abdominoperineal resection for low rectal cancer. Br J Surg 1985; 72: 595–598. [DOI] [PubMed] [Google Scholar]
- 6.Williams NS, Johnston D. Survival, recurrence after sphincter saving resection and abdominoperineal resection for carcinoma of the middle third of the rectum. Br J Surg 1984; 71: 278–282. [DOI] [PubMed] [Google Scholar]
- 7.Jones PF, Thomson HJ. Long-term results of a consistent policy of sphincter preservation in the treatment of carcinoma of the rectum. Br J Surg 1982; 69: 564–568. [DOI] [PubMed] [Google Scholar]
- 8.Slanetz CA, Herter FP, Grinnell RS. Anterior resection versus abdominoperineal resection for cancer of the rectum and rectosigmoid. Am J Surg 1972; 123: 110–117. [DOI] [PubMed] [Google Scholar]
- 9.Heimann TM, Szporn A, Bolnick K, et al. Local recurrence following surgical treatment of rectal cancer: comparison of anterior and abdominoperineal resection. Dis Colon Rectum 1986; 29: 862–864. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Canton EA, Pazdur R. Adjuvant medical therapy for colorectal cancer. Surg Clin North Am 1997; 77: 211–228. [DOI] [PubMed] [Google Scholar]
- 11.Fleshman JW, Myerson RJ. Adjuvant radiation therapy for adenocarcinoma of the rectum. Surg Clin North Am 1997; 77: 15–25. [DOI] [PubMed] [Google Scholar]
- 12.Stockholm Rectal Cancer Study Group. Preoperative short-term radiation therapy in operable rectal carcinoma: a prospective randomized trial. Cancer 1990; 66: 49–55. [DOI] [PubMed] [Google Scholar]
- 13.Swedish Rectal Cancer Trial. Local recurrence rate in a randomised multicentre trial of preoperative radiotherapy compared with operation alone in resectable rectal carcinoma. Eur J Surg 1996; 162: 397–402. [PubMed] [Google Scholar]
- 14.Gerard A, Boyse M, Nordlinger B, et al. Preoperative radiotherapy as adjuvant treatment in rectal cancer: final results of a randomized study of the European Organization for Research and Treatment of Cancer. Ann Surg 1988; 208: 606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med 1985; 312: 1465–1472. [DOI] [PubMed] [Google Scholar]
- 16.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991; 324: 709–715. [DOI] [PubMed] [Google Scholar]
- 17.NIH Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA 1990; 264: 1444–1450. [PubMed] [Google Scholar]
- 18.McArdle CS, Hole D. Impact of variability among surgeons on postoperative morbidity and mortality and ultimate survival. Br Med J 1991; 302: 1501–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly JV, Hellinger FJ. Physician, hospital factors associated with mortality of surgical patients. Med Care 1986; 24: 785–800. [DOI] [PubMed] [Google Scholar]
- 20.Steele RJC. The influence of surgeon case volume on outcome in site-specific cancer surgery. Eur J Surg Oncol 1996; 22: 211–213. [DOI] [PubMed] [Google Scholar]
- 21.Jarhult J. The importance of volume for outcome in cancer surgery-an overview. Eur J Surg Oncol 1996; 22: 205–210. [DOI] [PubMed] [Google Scholar]
- 22.Holm T, Johannson H, Cedermark B, et al. Influence of hospital- and surgeon-related factors on outcome after treatment of rectal cancer with or without preoperative radiotherapy. Br J Surg 1997; 84: 657–663. [PubMed] [Google Scholar]
- 23.Porter, GA, Soskolne CL, Yakimets WW, et al. Surgeon-related factors and outcome in rectal cancer. Ann Surg 1998; 227: 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steele GD Jr. The National Cancer Data Base report on colorectal cancer. Cancer 1994; 74: 1979–1989. [DOI] [PubMed] [Google Scholar]
- 25.Beart RW, Steele GD Jr, Menck HR, et al. Management and survival of patients with adenocarcinoma of the colon and rectum: a national survey of the Commission on Cancer. J Am Coll Surg 1995; 181: 225–236. [PubMed] [Google Scholar]
- 26.Cress R, Davidson-Allen K, Caggiano V. Cancer incidence and mortality in the Sacramento region 1988–1996. Sacramento, CA: Cancer Surveillance Program, Region 3; 1999.
- 27.Morris C, Cohen R, Perkins CL, et al. Cancer in California: 1988–1996. Sacramento, CA: California Department of Health Services, Cancer Surveillance Section; 1999.
- 28.American Joint Committee on Cancer. The manual for staging of cancer. Philadelphia: JB Lippincott; 1988: 145–50.
- 29.Seiffert J, ed. SEER Program: comparative staging guide for cancer, version 1.1. NIH Publication No. 93–3640. National Cancer Institute; 1993.
- 30.Cress R, Davidson-Allen K. Conversion from SEER extent of disease to AJCC stage grouping. Journal of Registry Management 1999; 26: 89–91. [Google Scholar]
- 31.California Cancer Registry. Cancer reporting in California. abstracting and coding procedures for hospitals. California Cancer Reporting System Standards, 1998.
- 32.Enker WE. Sphincter-preserving operations for rectal cancer. Oncology 1996; 10: 1673–1689. [PubMed] [Google Scholar]
- 33.American Medical Association. AMA physician select. AMA 1999. [Google Scholar]
- 34.American Hospital Association. AHA guide to the health care field. Chicago: AHA; 1994: 44–79.
- 35.American Hospital Association. AHA guide to the health care field. Chicago: AHA; 1995–96: 36–67.
- 36.American Medical Association. Graduate medical education directory. Chicago: AMA; 1994–95: 862–908.
- 37.American Medical Association. Graduate medical education directory. Chicago: AMA; 1996–97: 926–967.
- 38.Zaheer S, Pemberton JH, Farouk R, et al. Surgical treatment of adenocarcinoma of the rectum. Ann Surg 1998; 227: 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavery IC, Lopez-Kostner F, Fazio VW, et al. Chances of cure are not compromised with sphincter-saving procedures for cancer of the lower third of the rectum. Surgery 1997; 122: 779–785. [DOI] [PubMed] [Google Scholar]
- 40.Amato A, Pescatori M, Butti A. Local recurrence following abdominoperineal excision and anterior resection for rectal carcinoma. Dis Colon Rectum 1991; 34: 314–322. [DOI] [PubMed] [Google Scholar]
- 41.Fick TE, Baeten CG, von Meyenfeldt MF, et al. Recurrence and survival after abdominoperineal and low anterior resection for rectal cancer, without adjunctive therapy. Eur J Surg Oncol 1990; 16: 105–108. [PubMed] [Google Scholar]