Abstract
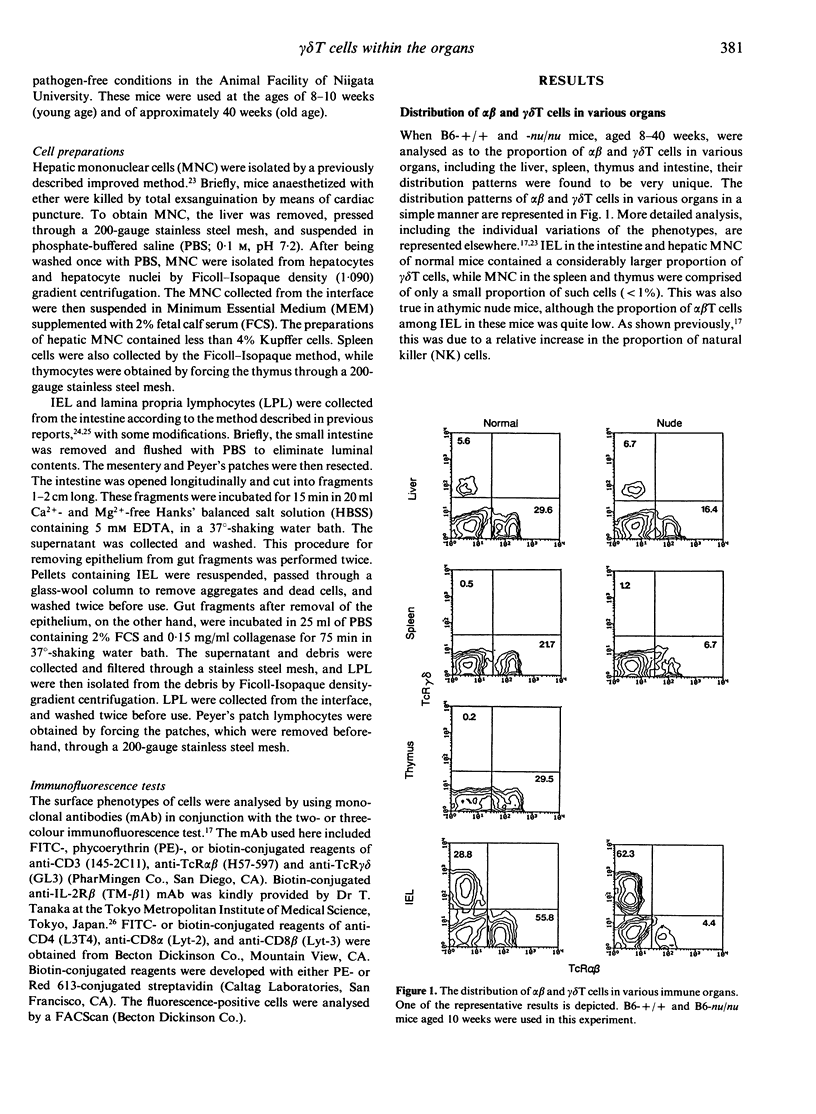
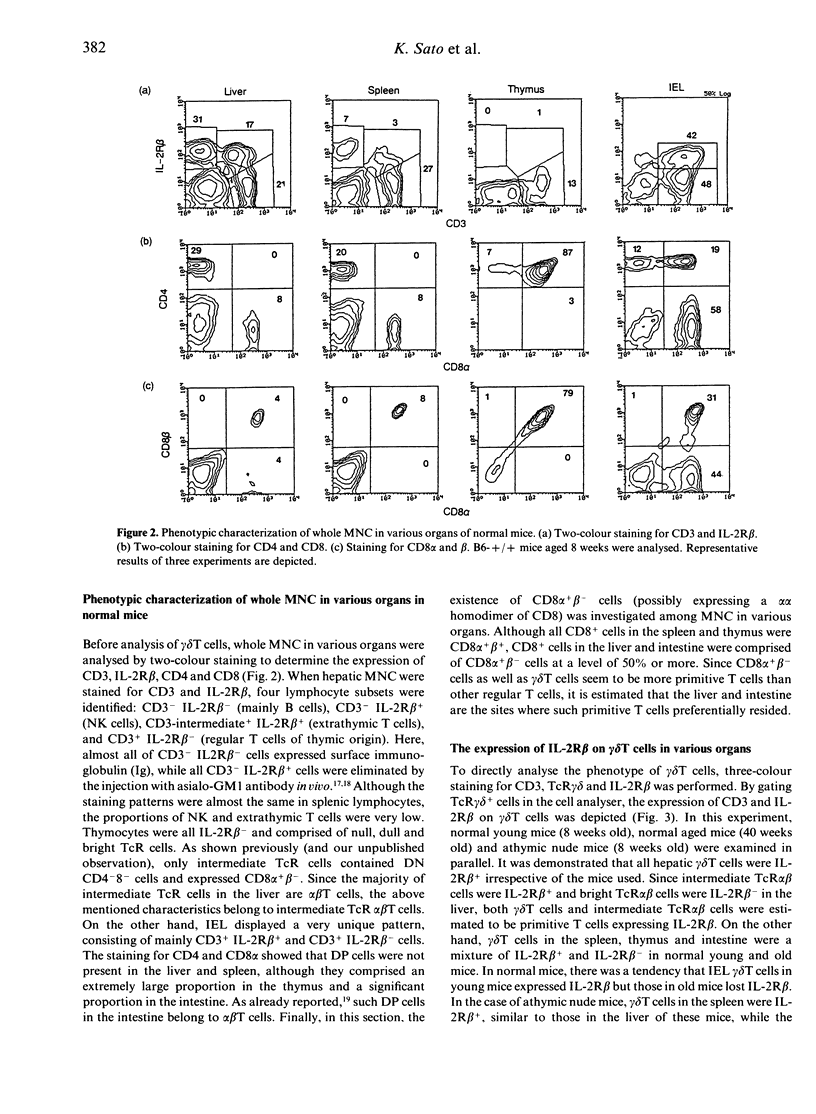
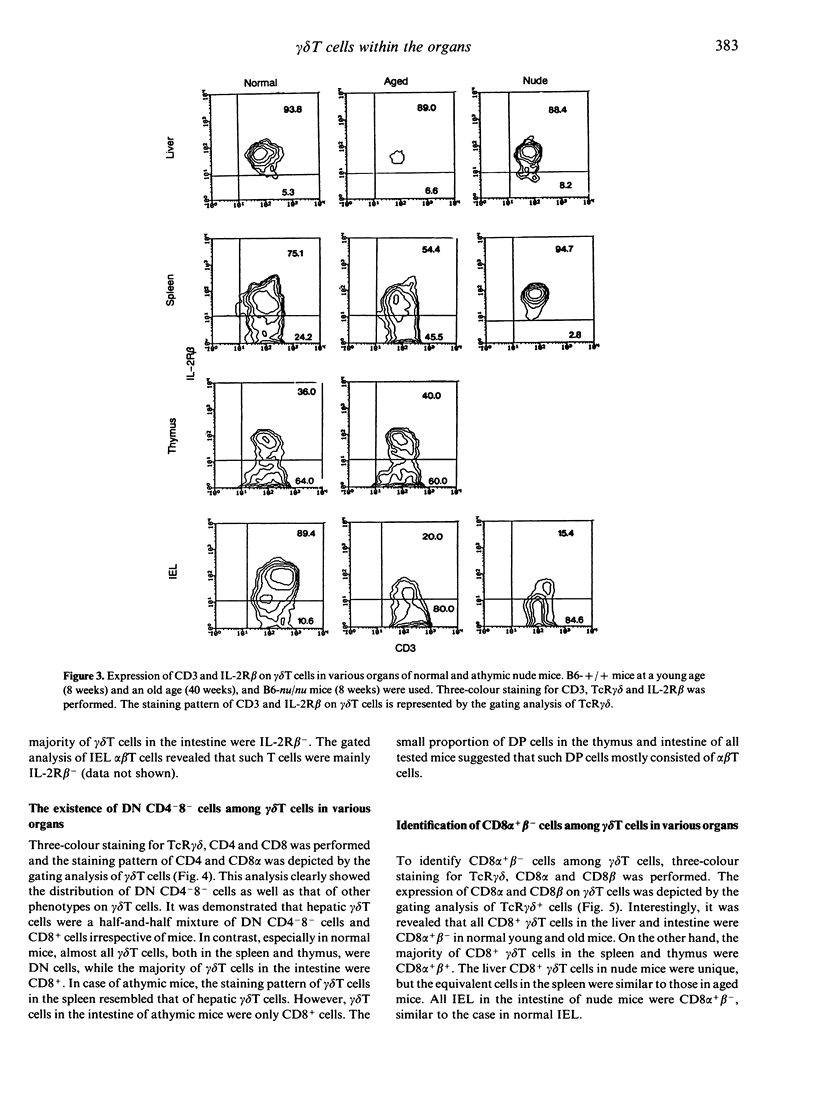
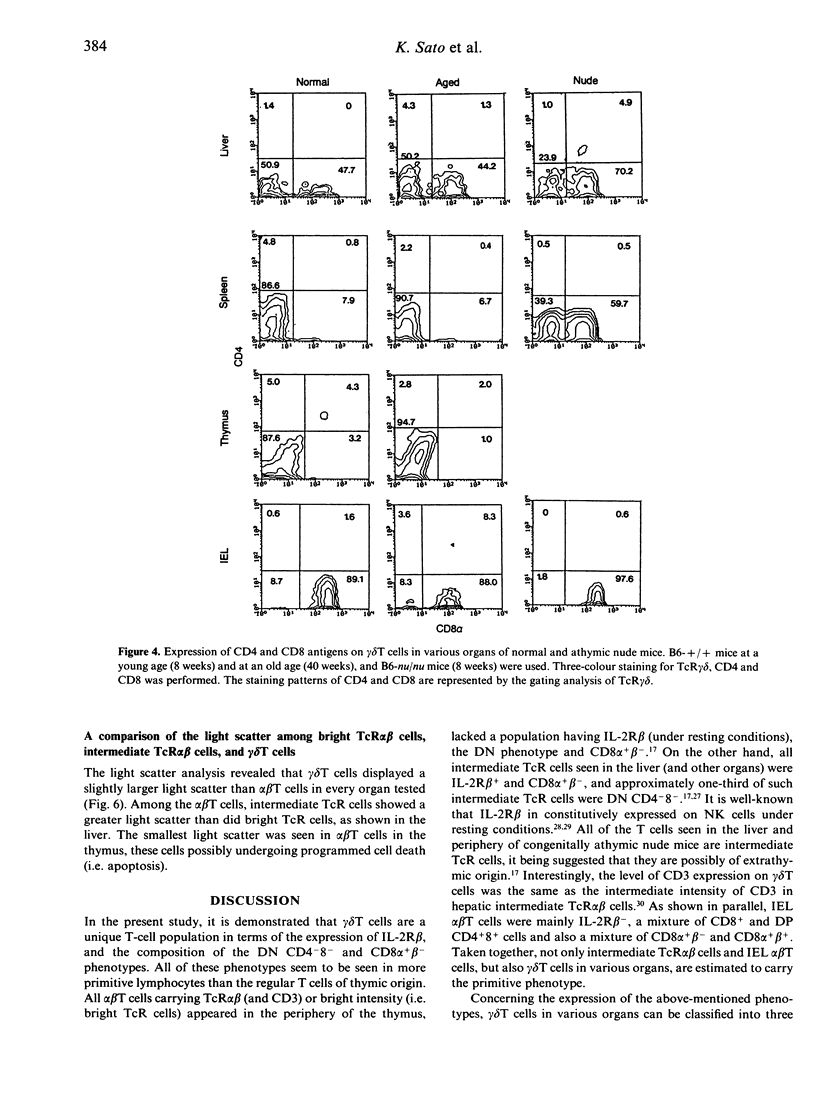
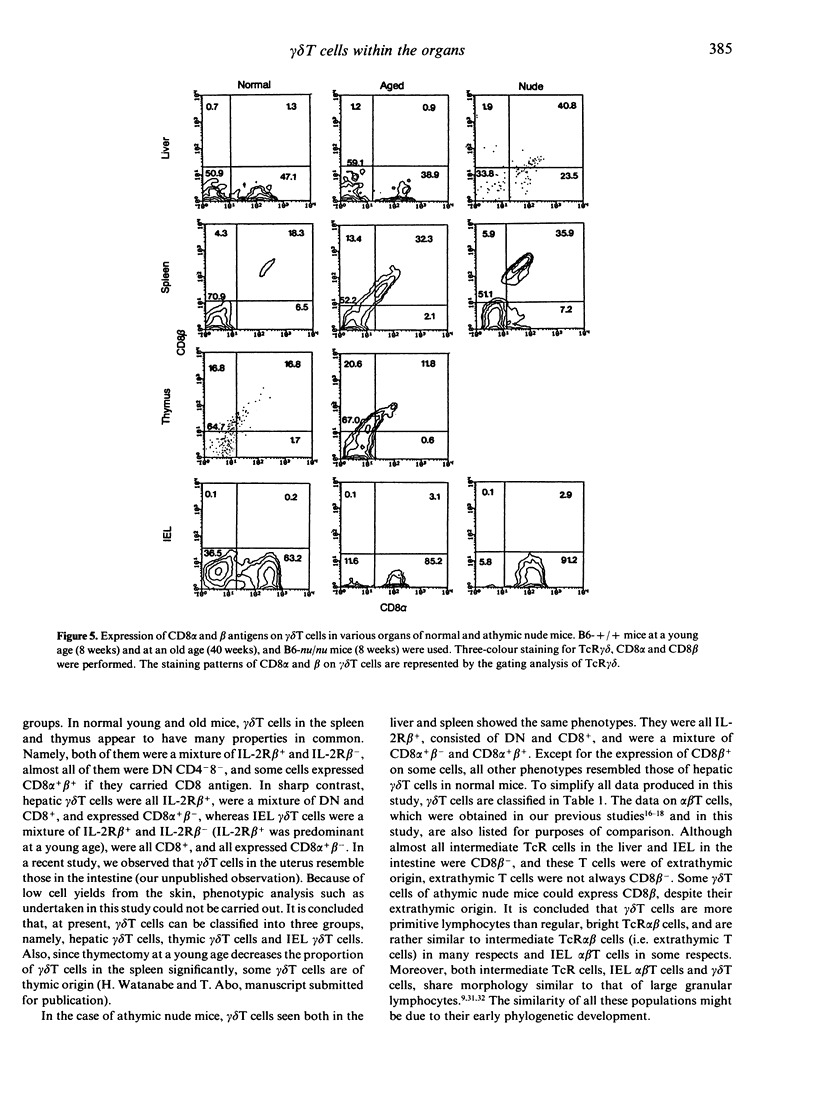
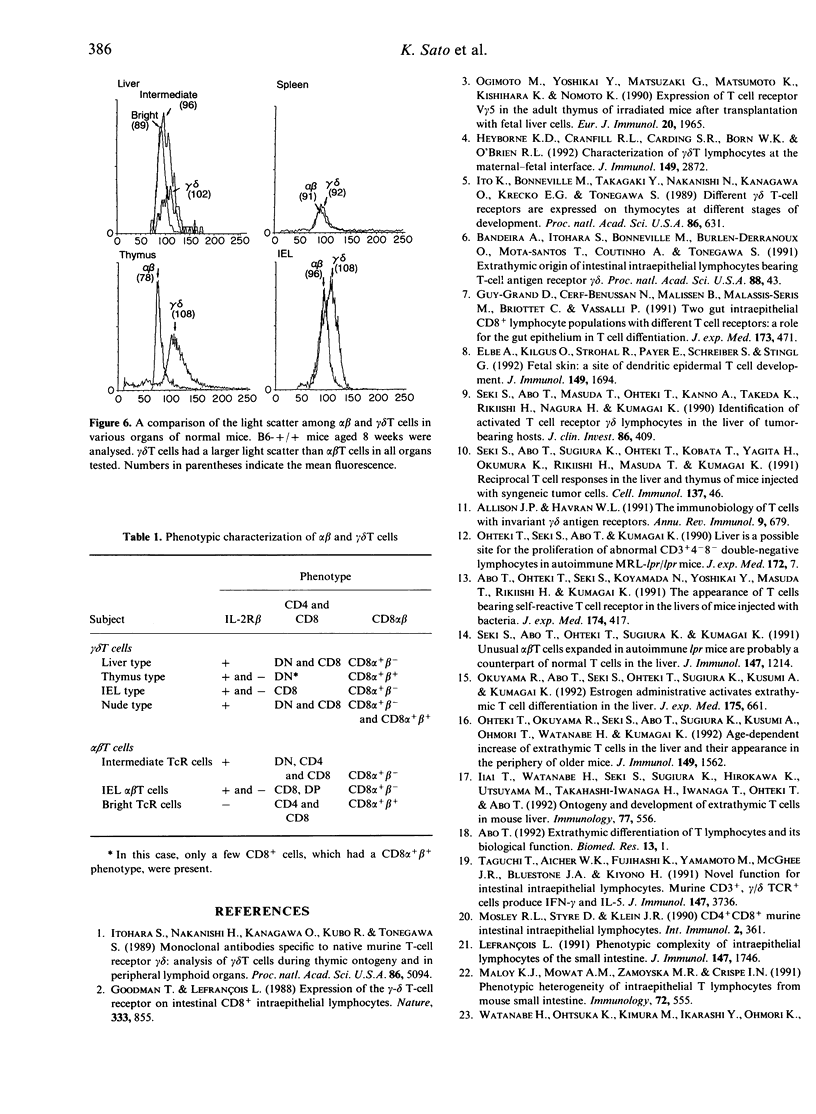
gamma delta T cells are known to localize preferentially in the epithelial regions and the hepatic sinusoids, and exhibit highly restricted V gene usage depending on their location. In the present study, gamma delta T cells in mice were further characterized in terms of their expression of the interleukin-2 receptor beta-chain (IL-2R beta), CD4 and CD8, and CD8 alpha and beta. This experiment was arranged to investigate whether gamma delta T cells have different properties depending on the organs and how gamma delta T cells are different from extrathymic alpha beta T cells, i.e. alpha beta T cells in the liver and intraepithelial lymphocytes in the intestine, in terms of the above phenotypes. Three-colour immunofluorescence tests using monoclonal antibodies revealed that gamma delta T cells can be classified into three groups: gamma delta T cells of the liver type are all IL-2R beta+, are comprised of double-negative (DN) CD8-CD4- and single-positive CD8+ (no CD4+) cells, and express CD8 alpha+ beta-; gamma delta T cells of the thymus type are a mixture of IL-2R beta+ and IL-2R beta-, are mainly DN, and express CD8 alpha+ beta+ if they carry CD8 antigens; and gamma delta T cells of the intestine type are also IL-2R beta+ or IL-2R beta-, are all CD8+, and express CD8 alpha+ beta-. gamma delta T cells in the spleen of normal mice are of the thymus type, while gamma delta T cells in the spleen of athymic nude mice seem to be of the liver type. All these properties of gamma delta T cells resemble those of extrathymic alpha beta T cells rather than regular alpha beta T cells of thymic origin. The present results reveal that gamma delta T cells and other extrathymic alpha beta T cells have many properties in common as primitive lymphocytes in phylogenetic development.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Ohteki T., Seki S., Koyamada N., Yoshikai Y., Masuda T., Rikiishi H., Kumagai K. The appearance of T cells bearing self-reactive T cell receptor in the livers of mice injected with bacteria. J Exp Med. 1991 Aug 1;174(2):417–424. doi: 10.1084/jem.174.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison J. P., Havran W. L. The immunobiology of T cells with invariant gamma delta antigen receptors. Annu Rev Immunol. 1991;9:679–705. doi: 10.1146/annurev.iy.09.040191.003335. [DOI] [PubMed] [Google Scholar]
- Arancia G., Malorni W., Iosi F., Zarcone D., Cerruti G., Favre A., Zeromski J., Grossi C. E., Moretta A. Morphological features of cloned lymphocytes expressing gamma/delta T cell receptors. Eur J Immunol. 1991 Jan;21(1):173–178. doi: 10.1002/eji.1830210126. [DOI] [PubMed] [Google Scholar]
- Davies M. D., Parrott D. M. Preparation and purification of lymphocytes from the epithelium and lamina propria of murine small intestine. Gut. 1981 Jun;22(6):481–488. doi: 10.1136/gut.22.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbe A., Kilgus O., Strohal R., Payer E., Schreiber S., Stingl G. Fetal skin: a site of dendritic epidermal T cell development. J Immunol. 1992 Sep 1;149(5):1694–1701. [PubMed] [Google Scholar]
- Goodman T., Lefrançois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988 Jun 30;333(6176):855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Cerf-Bensussan N., Malissen B., Malassis-Seris M., Briottet C., Vassalli P. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med. 1991 Feb 1;173(2):471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978 Dec 1;148(6):1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyborne K. D., Cranfill R. L., Carding S. R., Born W. K., O'Brien R. L. Characterization of gamma delta T lymphocytes at the maternal-fetal interface. J Immunol. 1992 Nov 1;149(9):2872–2878. [PubMed] [Google Scholar]
- Ito K., Bonneville M., Takagaki Y., Nakanishi N., Kanagawa O., Krecko E. G., Tonegawa S. Different gamma delta T-cell receptors are expressed on thymocytes at different stages of development. Proc Natl Acad Sci U S A. 1989 Jan;86(2):631–635. doi: 10.1073/pnas.86.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara S., Nakanishi N., Kanagawa O., Kubo R., Tonegawa S. Monoclonal antibodies specific to native murine T-cell receptor gamma delta: analysis of gamma delta T cells during thymic ontogeny and in peripheral lymphoid organs. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5094–5098. doi: 10.1073/pnas.86.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy K. J., Mowat A. M., Zamoyska R., Crispe I. N. Phenotypic heterogeneity of intraepithelial T lymphocytes from mouse small intestine. Immunology. 1991 Apr;72(4):555–562. [PMC free article] [PubMed] [Google Scholar]
- Mosley R. L., Styre D., Klein J. R. CD4+CD8+ murine intestinal intraepithelial lymphocytes. Int Immunol. 1990;2(4):361–365. doi: 10.1093/intimm/2.4.361. [DOI] [PubMed] [Google Scholar]
- Ogimoto M., Yoshikai Y., Matsuzaki G., Matsumoto K., Kishihara K., Nomoto K. Expression of T cell receptor V gamma 5 in the adult thymus of irradiated mice after transplantation with fetal liver cells. Eur J Immunol. 1990 Sep;20(9):1965–1970. doi: 10.1002/eji.1830200914. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Takeshita T., Nagata K., Mori S., Sugamura K. Differential expression of the IL-2 receptor subunits, p55 and p75 on various populations of primary peripheral blood mononuclear cells. J Immunol. 1989 Dec 1;143(11):3548–3555. [PubMed] [Google Scholar]
- Ohteki T., Okuyama R., Seki S., Abo T., Sugiura K., Kusumi A., Ohmori T., Watanabe H., Kumagai K. Age-dependent increase of extrathymic T cells in the liver and their appearance in the periphery of older mice. J Immunol. 1992 Sep 1;149(5):1562–1570. [PubMed] [Google Scholar]
- Ohteki T., Seki S., Abo T., Kumagai K. Liver is a possible site for the proliferation of abnormal CD3+4-8- double-negative lymphocytes in autoimmune MRL-lpr/lpr mice. J Exp Med. 1990 Jul 1;172(1):7–12. doi: 10.1084/jem.172.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama R., Abo T., Seki S., Ohteki T., Sugiura K., Kusumi A., Kumagai K. Estrogen administration activates extrathymic T cell differentiation in the liver. J Exp Med. 1992 Mar 1;175(3):661–669. doi: 10.1084/jem.175.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S., Abo T., Masuda T., Ohteki T., Kanno A., Takeda K., Rikiishi H., Nagura H., Kumagai K. Identification of activated T cell receptor gamma delta lymphocytes in the liver of tumor-bearing hosts. J Clin Invest. 1990 Aug;86(2):409–415. doi: 10.1172/JCI114726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S., Abo T., Ohteki T., Sugiura K., Kumagai K. Unusual alpha beta-T cells expanded in autoimmune lpr mice are probably a counterpart of normal T cells in the liver. J Immunol. 1991 Aug 15;147(4):1214–1221. [PubMed] [Google Scholar]
- Seki S., Abo T., Sugiura K., Ohteki T., Kobata T., Yagita H., Okumura K., Rikiishi H., Masuda T., Kumagai K. Reciprocal T cell responses in the liver and thymus of mice injected with syngeneic tumor cells. Cell Immunol. 1991 Oct 1;137(1):46–60. doi: 10.1016/0008-8749(91)90055-g. [DOI] [PubMed] [Google Scholar]
- Taguchi T., Aicher W. K., Fujihashi K., Yamamoto M., McGhee J. R., Bluestone J. A., Kiyono H. Novel function for intestinal intraepithelial lymphocytes. Murine CD3+, gamma/delta TCR+ T cells produce IFN-gamma and IL-5. J Immunol. 1991 Dec 1;147(11):3736–3744. [PubMed] [Google Scholar]
- Tanaka T., Tsudo M., Karasuyama H., Kitamura F., Kono T., Hatakeyama M., Taniguchi T., Miyasaka M. A novel monoclonal antibody against murine IL-2 receptor beta-chain. Characterization of receptor expression in normal lymphoid cells and EL-4 cells. J Immunol. 1991 Oct 1;147(7):2222–2228. [PubMed] [Google Scholar]
- van der Heijden P. J., Stok W., Bianchi A. T. Contribution of immunoglobulin-secreting cells in the murine small intestine to the total 'background' immunoglobulin production. Immunology. 1987 Dec;62(4):551–555. [PMC free article] [PubMed] [Google Scholar]


