Abstract
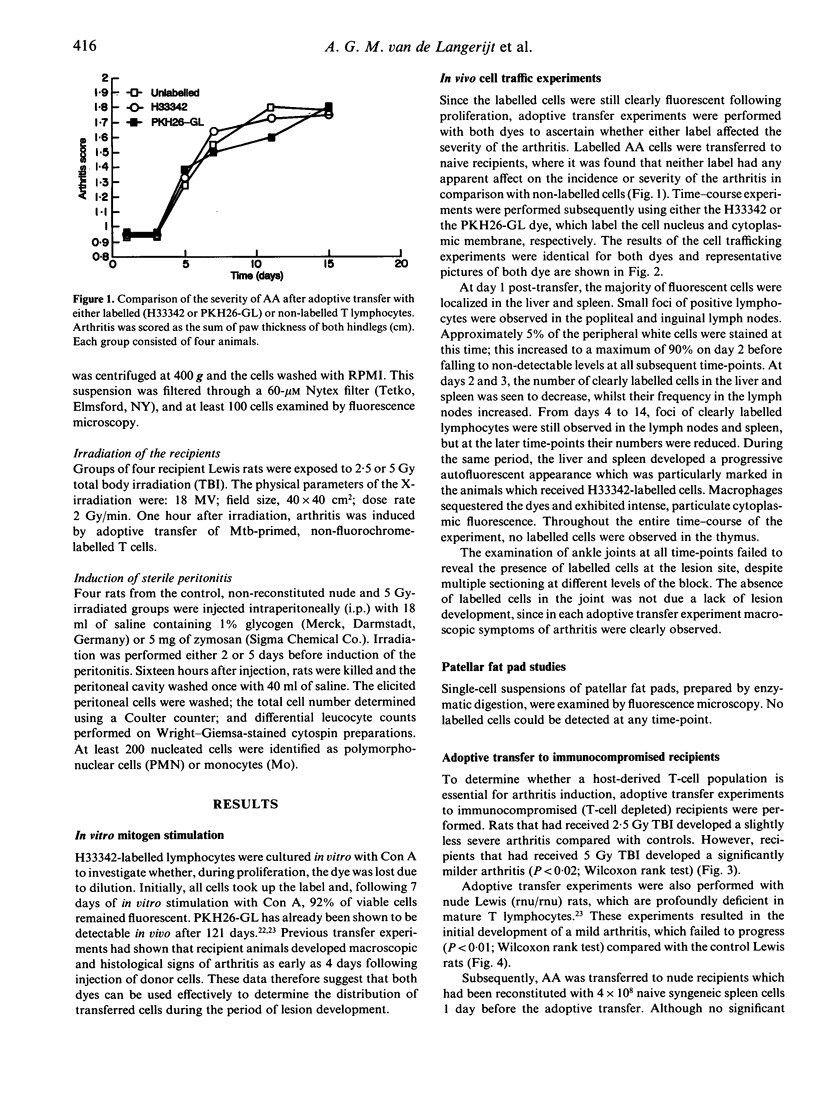
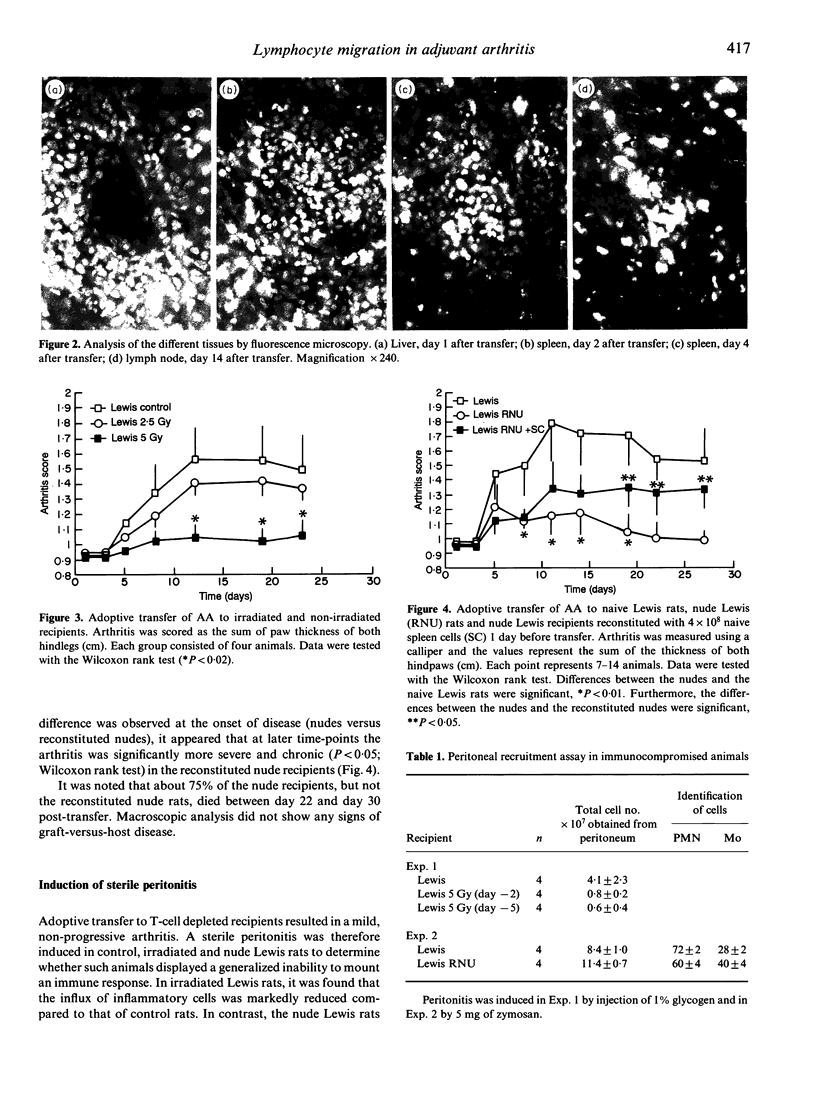
Adjuvant arthritis (AA) can be induced in Lewis rats by a single injection of either heat-killed Mycobacterium tuberculosis or the lipoidal amine CP20961. Concanavalin A (Con A)-stimulated T cells isolated from AA rats are able to adoptively transfer the disease to naive syngeneic recipients. It is unclear, however, whether these transferred cells traffic directly to the joint and initiate arthritis, or whether secondary host cells are responsible for activation of the disease. In the current investigation, T cells labelled with the vital fluorescent dyes Hoechst H33342 and Zynaxis PKH26-G were used to adoptively transfer adjuvant disease to naive recipients. At various stages of disease development sections of ankle joints, together with a range of soft tissues, were examined by fluorescence microscopy to determine the distribution of labelled donor cells in the recipients. Intensely fluorescent lymphocytes were observed in the liver, spleen and lymph nodes within 24 hr of adoptive transfer. Foci of such cells were clearly visible in the primary lymphoid tissues as late as 14 days after transfer. However, close examination of both ankle joint sections and patellar fat pad cells throughout the time-course of the study failed to detect any labelled cells at the lesion site. To develop these observations further, we performed adoptive transfers to nude Lewis rats (rnu/rnu) and found that they were only moderately sensitive and developed, at best, a transient arthritis. This observed difference could not be explained by a generalized lack of an inflammatory response, since we were able to elicit a zymosan peritonitis in the nude rats. However, in nude Lewis rats a striking increase in adoptively transferred AA was obtained after reconstitution with 4 x 10(8) naive syngeneic spleen cells. These combined observations suggest that a host-derived immune cell population is crucial for arthritis induction in the adoptive transfer system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Nun A., Wekerle H., Cohen I. R. Vaccination against autoimmune encephalomyelitis with T-lymphocyte line cells reactive against myelin basic protein. Nature. 1981 Jul 2;292(5818):60–61. doi: 10.1038/292060a0. [DOI] [PubMed] [Google Scholar]
- Billingham M. E., Carney S., Butler R., Colston M. J. A mycobacterial 65-kD heat shock protein induces antigen-specific suppression of adjuvant arthritis, but is not itself arthritogenic. J Exp Med. 1990 Jan 1;171(1):339–344. doi: 10.1084/jem.171.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingham M. E., Hicks C., Carney S. Monoclonal antibodies and arthritis. Agents Actions. 1990 Jan;29(1-2):77–87. doi: 10.1007/BF01964727. [DOI] [PubMed] [Google Scholar]
- Brooks C. G., Webb P. J., Robins R. A., Robinson G., Baldwin R. W., Festing M. F. Studies on the immunobiology of rnu/rnu "nude" rats with congenital aplasia of the thymus. Eur J Immunol. 1980 Jan;10(1):58–65. doi: 10.1002/eji.1830100112. [DOI] [PubMed] [Google Scholar]
- Chang Y. H., Pearson C. M., Abe C. Adjuvant polyarthritis. IV. Induction by a synthetic adjuvant: immunologic, histopathologic, and other studies. Arthritis Rheum. 1980 Jan;23(1):62–71. doi: 10.1002/art.1780230111. [DOI] [PubMed] [Google Scholar]
- DeJoy S. Q., Ferguson-Chanowitz K., Oronsky A. L., Zabriskie J. B., Kerwar S. S. Studies on the homing of Mycobacterium-sensitized T lymphocytes to the synovium during passive adjuvant arthritis. Cell Immunol. 1990 Oct 1;130(1):195–203. doi: 10.1016/0008-8749(90)90173-o. [DOI] [PubMed] [Google Scholar]
- DeJoy S. Q., Ferguson K. M., Oronsky A. L., Kerwar S. S. Studies on the synergy between collagen and adjuvant arthritis in rats. Cell Immunol. 1988 Apr 15;113(1):117–129. doi: 10.1016/0008-8749(88)90011-1. [DOI] [PubMed] [Google Scholar]
- Hinrichs D. J., Humphres R. C. The response of the nude (athymic) rat to actively induced and adoptively transferred experimental allergic encephalomyelitis. J Immunol. 1983 Jul;131(1):4–5. [PubMed] [Google Scholar]
- Horan P. K., Melnicoff M. J., Jensen B. D., Slezak S. E. Fluorescent cell labeling for in vivo and in vitro cell tracking. Methods Cell Biol. 1990;33:469–490. doi: 10.1016/s0091-679x(08)60547-6. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B., Issekutz A. C. T lymphocyte migration to arthritic joints and dermal inflammation in the rat: differing migration patterns and the involvement of VLA-4. Clin Immunol Immunopathol. 1991 Dec;61(3):436–447. doi: 10.1016/s0090-1229(05)80014-5. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B., Stoltz J. M. Stimulation of lymphocyte migration by endotoxin, tumor necrosis factor, and interferon. Cell Immunol. 1989 Apr 15;120(1):165–173. doi: 10.1016/0008-8749(89)90184-6. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B., Stoltz J. M., van der Meide P. The recruitment of lymphocytes into the skin by T cell lymphokines: the role of gamma-interferon. Clin Exp Immunol. 1988 Jul;73(1):70–75. [PMC free article] [PubMed] [Google Scholar]
- Issekutz T. B., Stoltz J. M., vd Meide P. Lymphocyte recruitment in delayed-type hypersensitivity. The role of IFN-gamma. J Immunol. 1988 May 1;140(9):2989–2993. [PubMed] [Google Scholar]
- Issekutz T. B., Webster D. M., Stoltz J. M. Lymphocyte recruitment in vaccinia virus-induced cutaneous delayed-type hypersensitivity. Immunology. 1986 May;58(1):87–94. [PMC free article] [PubMed] [Google Scholar]
- Klausen B., Hougen H. P. Quantitative studies of lymphoid organs, blood and lymph in inbred athymic and euthymic LEW rats under germfree and specified-pathogen-free conditions. Lab Anim. 1987 Oct;21(4):342–347. doi: 10.1258/002367787781363318. [DOI] [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M., Tamaoki N., Tanaka A., Shimamura K., Ozawa A., Kotani S., Saito M., Hioki K. Role of thymus for N-acetyl muramyl-L-alanyl-D-isoglutamine-induced polyarthritis and granuloma formation in euthymic and athymic nude rats or in neonatally thymectomized rats. Infect Immun. 1981 Feb;31(2):758–766. doi: 10.1128/iai.31.2.758-766.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson P., Holmdahl R., Dencker L., Klareskog L. In vivo treatment with W3/13 (anti-pan T) but not with OX8 (anti-suppressor/cytotoxic T) monoclonal antibodies impedes the development of adjuvant arthritis in rats. Immunology. 1985 Nov;56(3):383–391. [PMC free article] [PubMed] [Google Scholar]
- Loeffler D., Ratner S. In vivo localization of lymphocytes labelled with low concentrations of Hoechst 33342. J Immunol Methods. 1989 Apr 21;119(1):95–101. doi: 10.1016/0022-1759(89)90385-2. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., Ben-Nun A., Holoshitz J., Reshef T., Frenkel A., Rosenberg M., Cohen I. R. T lymphocyte lines producing or vaccinating against autoimmune encephalomyelitis (EAE). Functional activation induces peanut agglutinin receptors and accumulation in the brain and thymus of line cells. Eur J Immunol. 1983 May;13(5):418–423. doi: 10.1002/eji.1830130513. [DOI] [PubMed] [Google Scholar]
- PEARSON C. M. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956 Jan;91(1):95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- Sedgwick J., Brostoff S., Mason D. Experimental allergic encephalomyelitis in the absence of a classical delayed-type hypersensitivity reaction. Severe paralytic disease correlates with the presence of interleukin 2 receptor-positive cells infiltrating the central nervous system. J Exp Med. 1987 Apr 1;165(4):1058–1075. doi: 10.1084/jem.165.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slezak S. E., Horan P. K. Fluorescent in vivo tracking of hematopoietic cells. Part I. Technical considerations. Blood. 1989 Nov 1;74(6):2172–2177. [PubMed] [Google Scholar]
- Taurog J. D., Sandberg G. P., Mahowald M. L. The cellular basis of adjuvant arthritis. I. Enhancement of cell-mediated passive transfer by concanavalin A and by immunosuppressive pretreatment of the recipient. Cell Immunol. 1983 Feb 1;75(2):271–282. doi: 10.1016/0008-8749(83)90325-8. [DOI] [PubMed] [Google Scholar]
- Taurog J. D., Sandberg G. P., Mahowald M. L. The cellular basis of adjuvant arthritis. II. Characterization of the cells mediating passive transfer. Cell Immunol. 1983 Aug;80(1):198–204. doi: 10.1016/0008-8749(83)90106-5. [DOI] [PubMed] [Google Scholar]
- Teare G. F., Horan P. K., Slezak S. E., Smith C., Hay J. B. Long-term tracking of lymphocytes in vivo: the migration of PKH-labeled lymphocytes. Cell Immunol. 1991 Apr 15;134(1):157–170. doi: 10.1016/0008-8749(91)90339-d. [DOI] [PubMed] [Google Scholar]
- Vos J. G., Berkvens J. M., Kruijt B. C. The athymic nude rat. I. Morphology of lymphoid and endocrine organs. Clin Immunol Immunopathol. 1980 Feb;15(2):213–228. doi: 10.1016/0090-1229(80)90032-x. [DOI] [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Nevo Z., Frenkel A., Klajman A., Cohen I. R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]



