Abstract
Objective
To determine whether selected clinicopathologic factors, including the extent of pathologic response to preoperative radiation and chemotherapy (RT ± chemo), have an impact on long-term recurrence-free survival (RFS) in patients with locally advanced primary rectal cancer after optimal multimodality therapy.
Summary Background Data
Although complete pathologic response to preoperative RT ± chemo has been detected in up to 30% of rectal cancers, its significance on long-term outcome has not been widely reported. Previous retrospective studies evaluating clinical outcome in patients with complete or near-complete pathologic response documented good prognosis in this population but were limited by median follow-up in the range of 2 to 3 years.
Methods
Sixty-nine patients with locally advanced (T3–4 and/or N1) primary rectal cancer were prospectively identified. All were treated at one institution with preoperative RT to the pelvis (at least 4,500 cGy). Forty patients received concurrent preoperative 5-fluorouracil-based chemotherapy and 27 received both pre- and postoperative chemotherapy. Patients underwent resection 4 to 7 weeks after completion of RT. TNM stage, angiolymphatic or perineural invasion, and extent of response to preoperative RT ± chemo were determined by pathologic evaluation. Adverse pathologic features were defined as the presence of angiolymphatic and/or perineural invasion. RFS at 5 years was determined by the Kaplan-Meier method.
Results
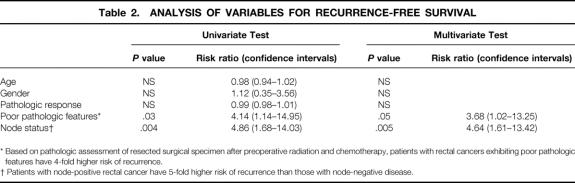
With a median follow-up of 69 months, 5-year RFS was 79%. RFS was significantly worse for patients with aggressive pathologic features and positive nodal status identified in the postirradiated surgical specimen. Risk ratios for RFS were 3.68 for the presence of aggressive pathologic features and 4.64 for node-positive rectal cancers. In patients with greater than 95% rectal cancer response to preoperative RT ± chemo, only one patient has died as a consequence of cancer, another has died of an unrelated cause, and the remainder were free of disease with a minimum follow-up of 47 months.
Conclusions
These data suggest that a marked response to preoperative RT ± chemo may be associated with good long-term outcome but was not predictive of RFS. The presence of poor histopathologic features and positive nodal status are the most important prognostic indicators after neoadjuvant therapy.
In patients with locally advanced (T3–4 and/or N1) rectal cancer, combined modality adjuvant therapy improves local control and survival. 1 Although the timing, dosage, and method of delivery are controversial, patients receiving preoperative radiation and chemotherapy (RT ± chemo) experience reduction of tumor bulk, enhanced sphincter preservation, improved functional results, and a lower rate of acute toxicity compared with patients receiving postoperative RT ± chemo. 2–5 Retrospective series and a single prospective study have also found improvement in overall and disease-free survival in patients with clinically resectable rectal cancer who received preoperative RT ± chemo. 4,6–8 A similar improvement was seen in patients who received preoperative RT alone. 9,10
Complete pathologic response after moderate- to high-dose preoperative RT for rectal cancer has been used as a surrogate endpoint for treatment outcome. In reported series, complete pathologic response rates range from 9% to 30%, 7,8,11,12 with an underlying assumption that a complete response correlates with a favorable long-term outcome. However, data to support this supposition are limited, particularly with respect to adequate follow-up. 13,14 Thus, the current analysis of consecutive patients with primary rectal cancer uniformly treated with preoperative RT was undertaken to determine whether clinicopathologic factors, including the extent of pathologic response to preoperative RT ± chemo, have an impact on long-term recurrence-free survival (RFS).
METHODS
During a 7-year period between 1987 and 1993, 171 consecutive patients with resectable primary rectal cancer were identified from the Memorial Sloan-Kettering Cancer Center (MSKCC) Colorectal Service and Department of Radiation Oncology databases. To ensure adequate long-term follow-up, patients treated after 1993 were excluded. Of these 171 patients, 34 did not undergo resection or received inadequate doses of radiation (< 4,500 cGy), 45 received preoperative RT at another institution, and in 4 data on preoperative RT were unavailable. An additional 14 were excluded because they received concurrent intraoperative radiation, and 5 could not be fully evaluated because paraffin blocks were not available for pathologic review. Therefore, 69 patients with potentially curable, locally advanced rectal cancer deemed eligible for preoperative RT ± chemo because of a bulky and/or tethered tumor or endorectal ultrasound evidence of T3–4 and/or N1 disease were analyzed.
All 69 study patients received preoperative external beam radiation to the pelvis consisting of at least 4,500 cGy delivered over 25 fractions and a boost to the primary tumor bed for a total radiation dose of 5,040 cGy in 28 fractions at 180 cGy per day according to previously described techniques. 7 Forty patients also received preoperative 5-fluorouracil (5-FU)-based chemotherapy, usually in combination with leucovorin, and 27 received both preoperative and postoperative chemotherapy. Agents and regimens were chosen based on a sequential group of phase 1 and 2 trials of concurrent 5-FU-based chemotherapy. Patients underwent complete surgical resection 4 to 7 weeks after completion of RT.
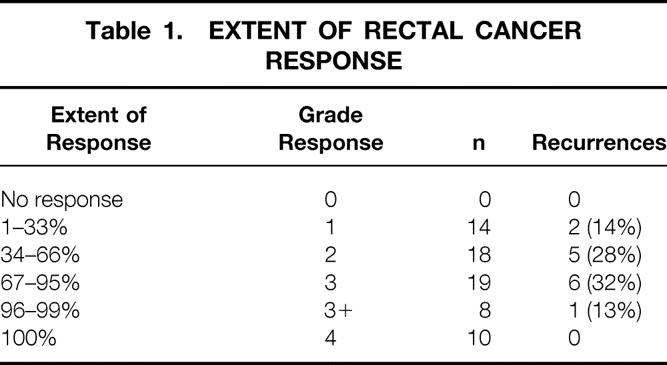
Pathologic evaluation was performed independently by two pathologists (S.T., D.S.K.) unaware of the clinical or radiologic findings. Discrepancies between the pathologists were resolved by consensus. Postirradiated resection specimens were evaluated for depth of tumor penetration, lymph node metastases, differentiation, and perineural and angiolymphatic invasion as well as extent of rectal cancer response to preoperative treatment. Assessment of treatment response was based on the examination of at least three microscopic sections of the tumor (unless the primary was small enough to be submitted entirely in fewer sections). Areas of tumor treatment effect were characterized by the replacement of neoplastic glands with loosely collagenized fibrous tissue and scattered chronic inflammatory cells (Fig. 1). Acellular mucin pools were found in cases of colloid carcinoma. Rectal cancer response was recorded as the percentage histologic response, ranging from no evidence of treatment effect (0%) to a complete response with no viable tumor identified (100%). A 5-point grading scale, developed by D.S.K. and validated as a measure of response in patients with non-small cell lung cancer receiving chemotherapy, 15 was used to categorize pathologic response to RT ± chemo. Tumors exhibiting no response were scored as 0, those with response in up to one third of the tumor as 1, those with response in between one third and two thirds as 2, those with response greater than two thirds but less than complete as 3, and those with complete response as 4 (Table 1). Tumors showing a near-complete response, characterized by microscopic foci of residual tumor consistent with greater than 95% response, were categorized as 3+.
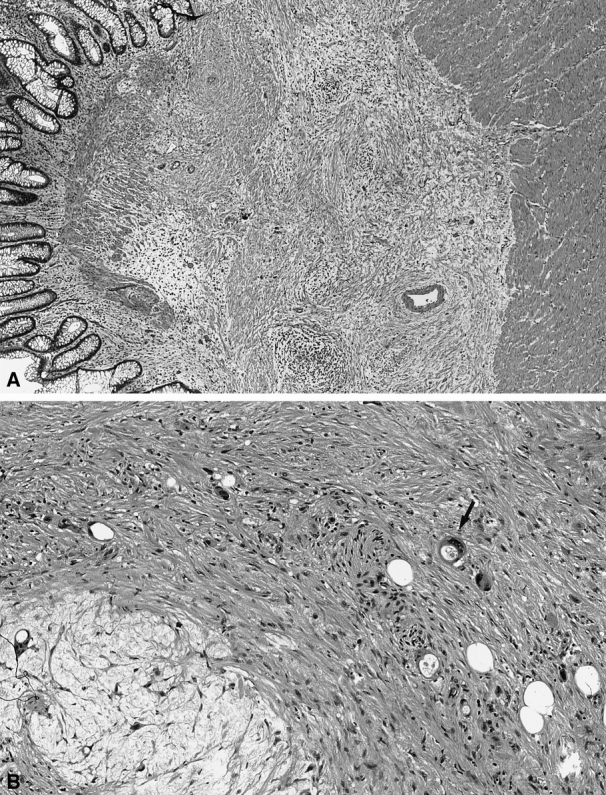
Figure 1. (A) Effect of preoperative radiation on rectal cancers was characterized by loosely collagenized fibrous tissue and scattered chronic inflammatory cells in place of neoplastic glands. (B) On high-power view, microscopic foci of residual cancer (arrow) are evident.
Table 1. EXTENT OF RECTAL CANCER RESPONSE
Of the 14 patients who developed recurrent disease during the follow-up interval, 4 had local recurrence, 8 had systemic recurrence, and 2 had both local and distant metastases. Recurrences were identified by clinical examination and imaging studies. Almost all (13/14) were confirmed histologically; the remaining recurrence was based on clinical and radiologic follow-up. Because of the small number of failures in this study population, all recurrences were analyzed together. RFS was determined by the Kaplan-Meier method. 16 Time to last follow-up, treatment failure, or death was measured from the date of surgery. Clinicopathologic variables, including patient age and gender, extent of pathologic response to preoperative RT ± chemo, presence of aggressive pathologic features (perineural and/or angiolymphatic invasion), and nodal status based on the postirradiated surgical specimen, were studied by univariate and multivariate analysis to identify factors predictive of clinical outcome. 17 For variables identified as independent prognostic factors on multivariate analysis, risk ratios were calculated.
RESULTS
Of the 69 patients available for analysis, 47 were men and 22 were women. Median age at surgery was 57 (range 24–79) years. Low anterior resection was possible in 47; 21 had an abdominoperineal resection; 1 underwent posterior pelvic exenteration. Median follow-up was 69 (range 3–140) months. The distribution of patients by extent of response to preoperative RT ± chemo is shown in Table 1. Among the 69 evaluable patients, 48 were alive without evidence of disease and 2 were alive with distant metastatic disease. Nineteen patients have died, 10 as a result of their rectal cancer. Estimated 5-year RFS for the entire study population was 79% (Fig. 2A).
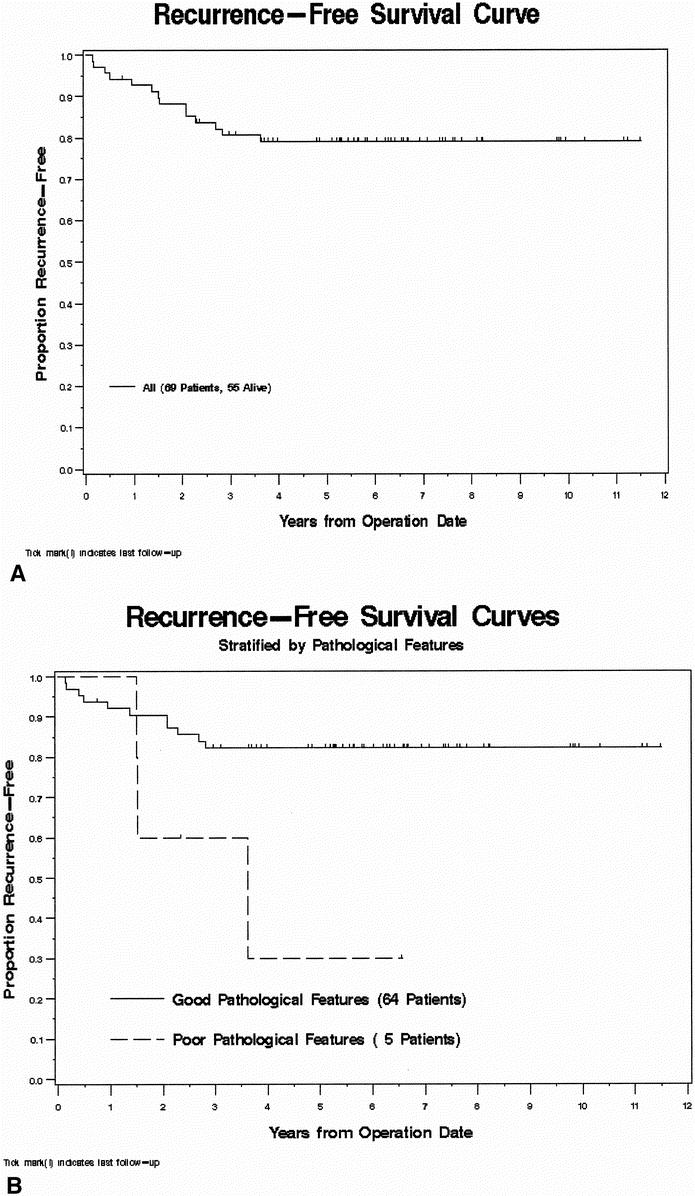
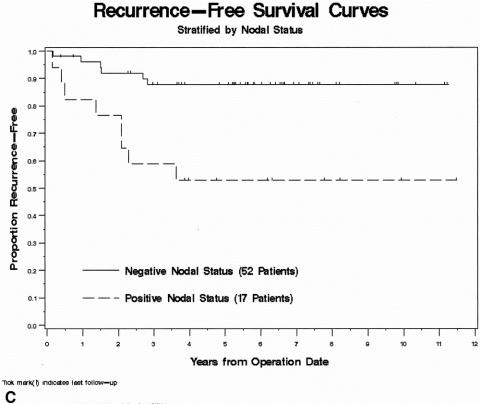
Figure 2. (A) Kaplan-Meier recurrence-free survival (RFS) curve for the study population (n = 69). (B) RFS stratified by the presence or absence of poor histopathologic features. (C) RFS stratified by the nodal status based on the postirradiated surgical specimen. Continued
Figure. Continued.
Patient specific factors studied, namely age and gender, were not predictive of RFS. Extent of response to preoperative RT ± chemo, whether analyzed as a categorical (grade response) or continuous (percentage response) variable, also did not have a significant impact on RFS. However, only one (6%) patient with greater than 95% rectal cancer response to preoperative RT ± chemo has developed recurrence. In this patient, widespread biopsy-proven osseous metastases were discovered within 2 months after surgery, and the patient died 3 months after resection. Of the remaining 17 patients with greater than 95% rectal cancer response to preoperative RT ± chemo, 1 died of causes unrelated to disease and the rest were all alive without evidence of disease at a minimum follow-up of 47 months.
The presence of adverse pathologic features and positive nodal status identified in the postirradiated rectal cancer surgical specimen were found to be statistically significant factors predictive for RFS by univariate and multivariate analysis (Table 2). In the presence of angiolymphatic and/or perineural invasion, 5-year RFS was 30% compared with 82% (P = .03) if these histologic features were absent (Fig. 2B). Similarly, 5-year RFS for patients with node-positive rectal cancer was significantly worse (53% vs. 89%, P = .004) than for those with node-negative disease (Fig. 2C). Risk ratios for RFS were 3.68 for the presence of aggressive pathologic features and 4.64 for node-positive rectal cancers.
Table 2. ANALYSIS OF VARIABLES FOR RECURRENCE-FREE SURVIVAL
* Based on pathologic assessment of resected surgical specimen after preoperative radiation and chemotherapy, patients with rectal cancers exhibiting poor pathologic features have 4-fold higher risk of recurrence.
† Patients with node-positive rectal cancer have 5-fold higher risk of recurrence than those with node-negative disease.
DISCUSSION
When previous studies with a reasonable sample size evaluated clinical outcome in patients with complete or near-complete tumor response, an excellent prognosis was observed at a median follow-up of 2 to 3 years. 13,14 Patients with resected rectal cancer with a complete pathologic response to preoperative RT ± chemo had 100% 5-year actuarial disease-free survival at a median follow-up of 26 months. 13 Similarly, patients with pT0N0 and pT2N0 rectal cancers treated by preoperative RT and curative surgical resection had 100% 5-year overall survival at a median follow-up of 33 months. 14 A more recent retrospective analysis in selected rectal cancers treated by preoperative RT and potentially curative surgery also found a significant survival advantage at a median follow-up of 43 months if the postirradiated surgical specimens exhibited complete tumor regression (pT0N0) or were early-stage tumors (pT1–2N0). 18 Although it is generally accepted that 80% of recurrences occur within 24 months, a recent study of patients receiving preoperative RT ± chemo showed that the median time to detection of local and distant recurrences was 34 and 24 months, respectively; almost 5 years of follow-up was required to detect 80% of all failures. 19 In the present study, designed to address long-term follow-up (median of 69 months) in a clinically homogeneous population uniformly treated at a single institution, 18 patients exhibited complete or near-complete (>95%) pathologic response to preoperative RT ± chemo. Actuarial 5-year RFS in this subset of patients was 94%. Of these 18 patients, only 1 had a documented recurrence, and this patient died with diffuse bone metastases 3 months after surgery. This most likely represents unrecognized synchronous metastases because the patient was noted, in retrospect, to have long-standing back pain.
Univariate and multivariate analyses showed significantly better 5-year RFS in patients with node-negative (N0) rectal cancer (89%) compared with those with node-positive disease (53%). Nodal metastases were less common in patients undergoing preoperative RT suggestive of a downstaging effect, 20 and this finding may, in part, account for improved survival in node-negative patients. The presence of poor pathologic features, consisting of angiolymphatic and/or perineural invasion, in the resected specimen was also predictive of RFS. Of the variables studied, calculated risk ratios suggested that nodal status in the postirradiated rectal cancer was the strongest predictor of RFS (see Table 2).
Although our results did not identify extent of rectal cancer response to preop RT ± chemo as an independent predictor of RFS, we believe that it may nevertheless reflect an important biologic factor. Almost all patients with greater than 95% rectal cancer response have survived without recurrence over an extended period of follow-up. The inability to show the prognostic significance of tumor response may be attributable to sample size, limited preoperative staging allowing for heterogeneity in pretreatment staging, and the possibility that the current pathologic evaluation for gauging rectal cancer response to preoperative RT ± chemo may be inadequate.
Infrequent disease failure in patients with a good response, which appears durable over a median follow-up beyond 5 years, suggests that novel strategies to enhance complete tumor regression by preoperative RT ± chemo are warranted. Current treatment modifications that may enhance the extent of pathologic response include hyperfractionated RT, 21–23 a delay of greater than 4 weeks between completion of RT and surgical resection, 24–26 and concomitant administration of chemotherapy. 6,8,12,25,27,28
In summary, our nonrandomized, retrospective analysis suggests that a marked rectal cancer response to preoperative RT ± chemo was clinically associated with fewer recurrences and good long-term outcome. The presence of adverse pathologic features (angiolymphatic and/or perineural invasion) was a significant prognostic indicator. However, nodal status in the postirradiated tumor specimen was the strongest factor predictive of clinical outcome. Based on the durability of our study results beyond a median follow-up of 5 years, patients with rectal cancer who undergo neoadjuvant therapy followed by complete surgical resection of a node-negative specimen can anticipate excellent long-term RFS. Ongoing treatment strategies aim to enhance complete response rates as well as accurately assess extent of tumor response to preoperative RT ± chemo via positron emission tomography imaging. 29 It is anticipated that these approaches may permit less radical surgery in patients who achieve a complete or near-complete response to preoperative RT ± chemo. In contrast, patients identified early on as nonresponders to one treatment protocol may benefit from treatment intensification or modification.
Footnotes
Correspondence: Jose G. Guillem, MD, Room C-1077, Colorectal Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021.
E-mail: guillemj@mskcc.org
Presented at the 36th Annual Meeting of the American Society of Clinical Oncology, New Orleans, LA, May 20–23, 2000.
Supported in part by National Cancer Institute Grant CA 82534-01 to J.G.G.
Accepted for publication December 5, 2001.
References
- 1.NIH Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA 1990; 264: 1444–1450. [PubMed] [Google Scholar]
- 2.Minsky BD, Cohen AM, Kemeny N, et al. Combined modality therapy of rectal cancer: decreased acute toxicity with the preoperative approach. J Clin Oncol 1992; 10: 1218–1224. [DOI] [PubMed] [Google Scholar]
- 3.Minsky BD, Coia L, Haller DG, et al. Radiation therapy for rectosigmoid and rectal cancer: results of the 1992–1994 Patterns of Care process survey. J Clin Oncol 1998; 16: 2542–2547. [DOI] [PubMed] [Google Scholar]
- 4.Valentini V, Coco C, Cellini N, et al. Preoperative chemoradiation for extraperitoneal T3 rectal cancer: acute toxicity, tumor response, and sphincter preservation. Int J Radiat Oncol Biol Phys 1998; 40: 1067–1075. [DOI] [PubMed] [Google Scholar]
- 5.Pucciarelli S, Friso ML, Toppan P, et al. Preoperative combined radiotherapy and chemotherapy for middle and lower rectal cancer: preliminary results. Ann Surg Oncol 2000; 7: 38–44. [DOI] [PubMed] [Google Scholar]
- 6.Chari RS, Tyler DS, Anscher MS, et al. Preoperative radiation and chemotherapy in the treatment of adenocarcinoma of the rectum. Ann Surg 1995; 221: 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grann A, Minsky BD, Cohen AM, et al. Preliminary results of preoperative 5-fluorouracil, low-dose leucovorin, and concurrent radiation therapy for clinically resectable T3 rectal cancer. Dis Colon Rectum 1997; 40: 515–522. [DOI] [PubMed] [Google Scholar]
- 8.Rich TA, Skibber JM, Ajani JA, et al. Preoperative infusional chemoradiation therapy for stage T3 rectal cancer. Int J Radiat Oncol Biol Phys 1995; 32: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 9.Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer [published erratum appears in N Engl J Med 1997; 336: 1539]. N Engl J Med 1997; 336: 980–987. [DOI] [PubMed] [Google Scholar]
- 10.Dahlberg M, Glimelius B, Pahlman L. Improved survival and reduction in local failure rates after preoperative radiotherapy: evidence for the generalizability of the results of Swedish Rectal Cancer Trial. Ann Surg 1999; 229: 493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohiuddin M, Marks G. High-dose preoperative irradiation for cancer of the rectum, 1976–1988. Int J Radiat Oncol Biol Phys 1991; 20: 37–43. [DOI] [PubMed] [Google Scholar]
- 12.Meterissian S, Skibber J, Rich T, et al. Patterns of residual disease after preoperative chemoradiation in ultrasound T3 rectal carcinoma. Ann Surg Oncol 1994; 1: 111–116. [DOI] [PubMed] [Google Scholar]
- 13.Willett CG, Warland G, Hagan MP, et al. Tumor proliferation in rectal cancer following preoperative irradiation. J Clin Oncol 1995; 13: 1417–1424. [DOI] [PubMed] [Google Scholar]
- 14.Kaminsky-Forrett MC, Conroy T, Luporsi E, et al. Prognostic implications of downstaging following preoperative radiation therapy for operable T3-T4 rectal cancer. Int J Radiat Oncol Biol Phys 1998; 42: 935–941. [DOI] [PubMed] [Google Scholar]
- 15.Rusch V, Klimstra D, Venkatraman E, et al. Aberrant p53 expression predicts clinical resistance to cisplatin-based chemotherapy in locally advanced non-small cell lung cancer. Cancer Res 1995; 55: 5038–5042. [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 17.Cox DR, Oakes D. Analysis of survival data, 4th ed. Chapman and Hall, 1990.
- 18.Luna-Perez P, Trejo-Valdivia B, Labastida S, et al. Prognostic factors in patients with locally advanced rectal adenocarcinoma treated with preoperative radiotherapy and surgery. World J Surg 1999; 23: 1069–1075. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad NR, Nagle D. Long-term results of preoperative radiation therapy alone for stage T3 and T4 rectal cancer. Br J Surg 1997; 84: 1445–1448. [PubMed] [Google Scholar]
- 20.Graf W, Dahlberg M, Osman MM, et al. Short-term preoperative radiotherapy results in down-staging of rectal cancer: a study of 1316 patients. Radiother Oncol 1997; 43: 133–137. [DOI] [PubMed] [Google Scholar]
- 21.Saunders MI, Dische S, Grosch EJ, et al. Experience with CHART. Int J Radiat Oncol Biol Phys 1991; 21: 871–878. [DOI] [PubMed] [Google Scholar]
- 22.Coucke PA, Sartorelli B, Cuttat JF, et al. The rationale to switch from postoperative hyperfractionated accelerated radiotherapy to preoperative hyperfractionated accelerated radiotherapy in rectal cancer. Int J Radiat Oncol Biol Phys 1995; 32: 181–188. [DOI] [PubMed] [Google Scholar]
- 23.Valdagni R. Altered fractionation in radiotherapy. Tumori 1998; 84: 155–159. [DOI] [PubMed] [Google Scholar]
- 24.Medical Research Council Rectal Cancer Working Party. Randomised trial of surgery alone versus radiotherapy followed by surgery for potentially operable locally advanced rectal cancer. Lancet 1996; 348: 1605–1610. [PubMed] [Google Scholar]
- 25.Berger C, de Muret A, Garaud P, et al. Preoperative radiotherapy (RT) for rectal cancer: predictive factors of tumor downstaging and residual tumor cell density (RTCD): prognostic implications. Int J Radiat Oncol Biol Phys 1997; 37: 619–627. [DOI] [PubMed] [Google Scholar]
- 26.Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90–01 randomized trial. J Clin Oncol 1999; 17: 2396. [DOI] [PubMed] [Google Scholar]
- 27.Janjan NA, Khoo VS, Abbruzzese J, et al. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys 1999; 44: 1027–1038. [DOI] [PubMed] [Google Scholar]
- 28.Bosset JF, Magnin V, Maingon P, et al. Preoperative radiochemotherapy in rectal cancer: long-term results of a phase II trial. Int J Radiat Oncol Biol Phys 2000; 46: 323–327. [DOI] [PubMed] [Google Scholar]
- 29.Guillem JG, Puig-La Calle J, Jr, Akhurst T, et al. Prospective assessment of primary rectal cancer response to preoperative radiation and chemotherapy using 18-fluorodeoxyglucose positron emission tomography. Dis Colon Rectum 2000; 43: 18–24. [DOI] [PubMed] [Google Scholar]