Abstract
Objective
To communicate results of laparoscopic treatment of pancreatic pseudocyst (PP) and resection of benign lesions of the pancreas. Perioperative data, surgical outcomes, techniques and insights from 54 cases are presented.
Summary Background Data
Although laparoscopic therapy for other solid organs has been widely adopted, reports of therapeutic laparoscopy of the pancreas have been few and of limited numbers, and its role in pancreatic disease is still unclear.
Methods
Eighteen men and 11 women were selected for laparoscopic PP surgery. Four distinct laparoscopic approaches were used. An additional 9 men and 16 women underwent laparoscopic distal pancreatectomy (LDP) using a technique similar to the lesser sac approach.
Results
Laparoscopic PP surgery was completed successfully in 28 of 29 patients. The overall mean operative time was 2.8 hours and the mean postoperative length of stay was 4.4 days. Of the techniques described, the authors prefer cyst gastrostomy by the lesser sac approach or the minilaparoscopic cystic gastrostomy. LDP was attempted in 25 patients and completed successfully in 23. One underwent a successful hand-assisted enucleation of an insulinoma. In 12 cases the spleen was preserved. Mean operative time was 3.7 hours, and mean postoperative length of stay was 4.1 days.
Conclusions
In the authors’ experience, minimally invasive treatment of PP produces good results and avoids difficulties linked with percutaneous drainage or endoscopic internal procedures. However, combining upper endoscopy with intragastric laparoscopic surgery offers advantages of both. LDP compares well to open procedures and often allows preservation of the spleen.
Laparoscopy was first used clinically to diagnose solid organ, abdominal disease. In independent accounts dating back to the turn of the 20th century, both Kelling and Jacobaeus described the earliest laparoscopies performed in patients with liver disease 1,2 in the presence of gross ascites. The practice of diagnostic laparoscopy for liver disease has evolved over the decades to a procedure often done under local anesthesia, where pneumoperitoneum has replaced hydroperitoneum. The role of laparoscopy in staging and diagnosis of pancreatic disease did not emerge for another 60 years. 3–5 The advance to therapeutic solid-organ laparoscopy has only recently occurred. Soon after the introduction of laparoscopic cholecystectomy, pioneering surgeons described successful laparoscopic resections of spleen, adrenal glands, and kidneys. As experience has accrued and favorable outcomes have been reported with these procedures, they have become widely adopted. Such has not been the case with laparoscopic pancreatic surgery. Reports of therapeutic laparoscopy of the pancreas have been few and of limited numbers. 4,6,7 Laparoscopic surgery of the pancreas remains in its infancy, and its role in pancreatic disease is still unclear. Although several laparoscopic pancreatic procedures have been described, debate continues over which procedures can be safely and adequately performed and which clearly benefit the patient when performed laparoscopically.
In this study, to our knowledge the largest series of therapeutic laparoscopic procedures yet reported, we review our experience with these techniques for 1) the treatment of pancreatic pseudocyst [PP] and 2) resection of benign lesions of the pancreas. Perioperative data, surgical outcomes, techniques, and insights will be presented.
PATIENTS AND METHODS
A total of 54 patients underwent an attempted therapeutic laparoscopic pancreatic procedure between May 1997 and January 2002 in one of two teaching centers (University of Kentucky Medical Center and Carolinas Medical Center) participating in this study. Twenty-nine patients with histories of acute or chronic pancreatitis who subsequently developed PP were selected to undergo laparoscopic treatment. Our experience suggests that patients with pancreatic pseudocysts that are large or not amenable (anatomically or technically) to endoscopic drainage should be considered candidates for a laparoscopic internal drainage procedure. As well, patients with neuroendocrine pancreatic tumors and cystic or other (presumed) benign masses in the body and tail of the pancreas may benefit from laparoscopic extirpation of such lesions. Etiologies of the pancreatitis in the patients selected included alcohol (10 patients), gall stones (14 patients), endoscopic retrograde cholangiopancreatography (2 patients), idiopathic conditions (2 patients), and hypercalcemia (1 patient). In all cases the patients had been followed clinically and with serial computed tomography (CT) scans to monitor for nonresolution, development of symptoms, and growth of their pseudocysts. Several of these patients were referred for surgery once they were ruled out as candidates for endoscopic drainage of their PP. Eighteen men and 11 women ranging in age from 25 to 78 years underwent laparoscopic PP surgery. Four different laparoscopic approaches (described below) were used to internally drain the pseudocysts. Nine patients underwent a lesser sac approach to a cyst-gastrostomy; 16 had an intra-gastric cyst-gastrostomy performed 5 of which were treated by a mini-laparoscopic (2 mm instruments) method; and 3 underwent cyst-jejunostomy. One patient had an external drainage performed.
Laparoscopic distal pancreatectomy (LDP) was attempted in the other 25 patients (9 men, 16 women), ranging in age from 26 to 73 years. All of these patients, presenting consecutively, had lesions (Table 1) confirmed or suspected in the neck, body, or tail of the pancreas. Seven patients were diagnosed with insulinomas and each underwent preoperative CT and/or magnetic resonance imaging (MRI) scanning to localize the lesions. Four of these patients also had preoperative selective arterial infusion of calcium with portal and hepatic vein sampling in a further attempt to localize a tumor that had not been identified by prior scans or ultrasounds.
Table 1. INDICATIONS FOR LDP
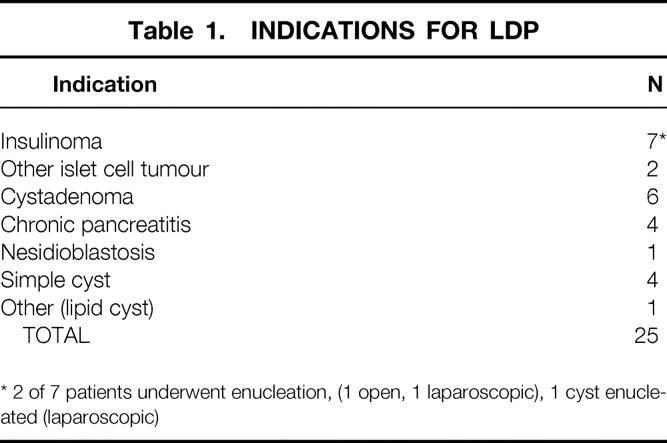
* 2 of 7 patients underwent enucleation, (1 open, 1 laparoscopic), 1 cyst enucleated (laparoscopic)
All patients who were considered for LDP were administered polyvalent pneumococcal, meningococcal, and Haemophilus influenzae vaccines at least 2 weeks before surgery, in anticipation of the possibility that preservation of the spleen might not be feasible. All patients in this series received perioperative antibiotics and deep vein thrombosis prophylaxis.
Operative Technique
Laparoscopic Pancreatic Pseudocyst Procedures
Four distinct laparoscopic techniques of cyst enteric drainage were employed through the course of this study. In decreasing order of frequency, they were 1) pancreatic cyst gastrostomy via the lesser sac approach, 2) minilaparoscopic pancreatic cyst gastrostomy, 3) intragastric pancreatic cyst gastrostomy, and 4) pancreatic cyst jejunostomy. As well, one patient underwent a laparoscopic necrosectomy and external drainage of an acute pseudocyst.
Cyst Gastrostomy by the Lesser Sac Approach.
This technique has been previously reported in detail, 4 so an abbreviated description follows. The patient is placed in the split leg or low lithotomy position. The surgeon stands between the patient’s legs. Four or five trocars are routinely used: a 5 or 10 mm camera port is placed in the supraumbilical rim, a 5 mm working port used for retraction is placed in subxiphoid area, and if further retraction or exposure is required a 5 mm port is then placed in the left subcostal region. Finally, an 11 mm working port through which the endovascular stapler can be introduced is inserted in the left upper quadrant.
A window is made in the gastrocolic omentum through which the lesser sac is entered. The stomach is elevated and a suitable site along the posterior gastric wall/cyst interface is selected (Fig. 1). A cystotomy is then made adjacent to the corresponding posterior gastric wall gastrotomy, and into this an endoscopic stapling device is introduced. When the device is fired, a hemostatic anastomosis is secured. The opening is then sutured.
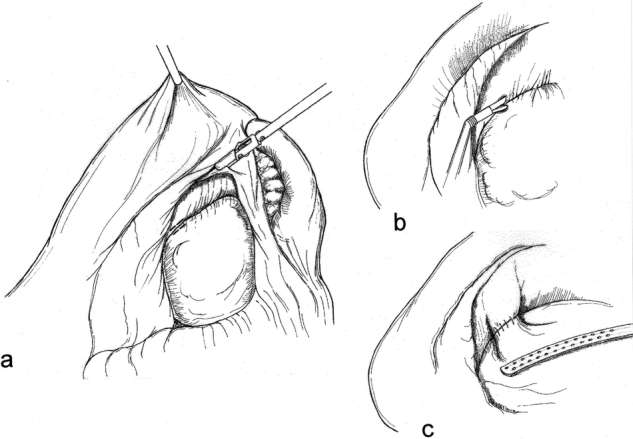
Figure 1. Lesser sac approach. After the lesser sac is opened (A), a cyst-gastrostomy is achieved by performing a pseudocystotomy and gastrotomy (B) and anastomising them with an endoscopic stapler (C).
The advantages of this approach include the avoidance of an anterior gastrotomy, as well as the assurance of a fairly robust anastomosis that is not dependent on the adherence of the cyst to the posterior gastric wall. Because the entire anastomosis is either stapled or sutured, hemostasis is ensured.
Minilaparoscopic Cystic Gastrostomy.
A gastroscope is introduced per os and situated in the stomach, which is insufflated and fully distended. Appropriate sites for trocar placement through the anterior abdominal wall are selected by gentle external palpation and endoscopic visualization of the corresponding indent. Two or three 2 mm ports are then inserted transabdominally into the gastric lumen under endoscopic visualization. Intermittently throughout the operation, a 2 mm laparoscope is inserted through one of the ports. A combination of both endoscopic and minilaparoscopic imaging provides excellent visualization (Fig. 2). The location of the pseudocyst bulging through the posterior gastric wall is confirmed with instrument palpation and/or aspiration, using a 22-gauge spinal needle. A gastrostomy is made through the posterior gastric wall into the pseudocyst using a minihook cautery. Return of copious viscous fluid is usually encountered. The nasogastric tube, initially positioned above the gastroesophageal junction, is placed to suction. The cyst fluid can also be aspirated via the endoscope.
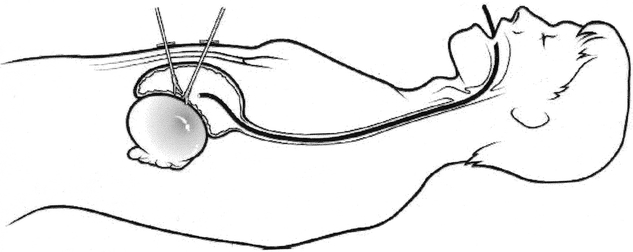
Figure 2. Mini-laparoscopic cyst-gastrostomy. An endoscope is utilized for vision and insufflation. The back wall of the stomach and the anterior wall of the pseudocyst are opened with 2 mm instruments, which have been passed directly into the stomach through the abdominal wall.
After a biopsy specimen of the cyst wall is obtained, the cyst gastrostomy is extended to 4 cm. Hemostasis is secured using electrocautery. The pseudocyst cavity can be examined and explored using the endoscope. The debris within the cyst cavity can be removed using both the minilaparoscopic instruments and endoscopic snares. If necessary the borders of the cyst gastroscopy may be sutured, using a minilaparoscopic intragastric technique.
Finally, the nasogastric tube is placed into the stomach and through the cyst gastrostomy into the cyst cavity. The minilaparoscopic instruments are then removed from the stomach. The ports remain through the abdominal wall, allowing access to the peritoneal cavity. A minilaparoscopic survey of the abdominal cavity is then carried out, during which the anterior gastric wall puncture sites are visualized. If indicated they may be closed with silk sutures. The nasogastric tube remains in site postoperatively for a 24-hour period, and on removal of the tube the patient is placed on a liquid diet.
Transgastric Cyst Gastrostomy.
In the performance of a transgastric cyst gastrostomy, the patient is again placed in lithotomy position with the surgeon standing between the legs. After peritoneal access is obtained at the umbilicus, a gastroscope is introduced into the stomach. While pneumoperitoneum is maintained at low levels (5–8 mm Hg), the stomach is insufflated via the gastroscope. Using both intraperitoneal and intragastric visualization, two or three laparoscopic trocars are inserted directly through the abdominal wall into the stomach. These are spaced several centimeters apart along the greater curve of the stomach.
Two particular types of trocars are most often used in this procedure. Balloon-tipped trocars are essentially 11 mm in diameter. After perforating the gastric wall, the trocar’s balloon can be inflated within the stomach. This prevents leakage of air or intragastric contents into the peritoneal cavity, keeps the trocars within the stomach, and holds the stomach against the abdominal wall. The radially dilating trocar (Innerdyme, Inc.) is also effective for intragastric surgery. These trocars work through a sheath that is initially placed into the stomach over a Veress needle. The sheath expands as the actual working trocar is placed through it and helps keep the stomach from slipping off the trocar during the operation. An important consideration is the mode of insufflation of the stomach. Judicious insufflation with the endoscope is recommended versus insufflation through one of the trocars. Discretion must be used to avoid severe gaseous distention of the small intestine.
Throughout the procedure vision is maintained with the gastroscope and, if a third port is entered, with the laparoscope. The bulging pseudocyst is located through the posterior gastric wall with palpation or with a long 22-gauge spinal needle. A gastrotomy is made through the posterior gastric wall into the pseudocyst, utilizing electrosurgery or, more recently, ultrasonic dissection (Fig. 3). The cyst wall is biopsied and sent for frozen section. After complete drainage of the pseudocyst, the cyst gastrostomy is extended to 4 cm and is often sutured, using 2-0 silk or absorbable sutures. At the completion of the intragastric portion of the operation, a nasogastric tube can be fed down through the stomach and into the pseudocyst.
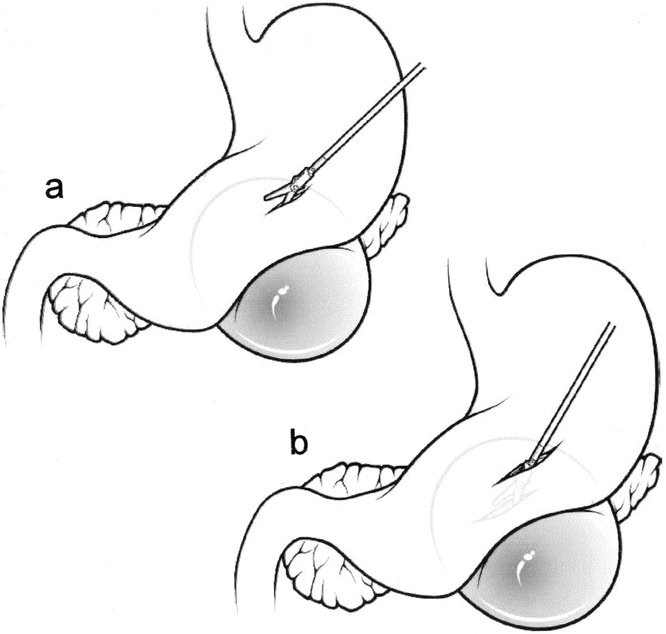
Figure 3. Intragastric pancreatic cyst gastrostomy. Standard laparoscopic instruments are used to perform an anterior gastrotomy. Through the gastrotomy the posterior wall of the stomach (A) and the anterior wall of the pseudocyst (B) are opened.
If balloon trocars are used in the operation, they are then desufflated, and the trocars are pulled from the gastric wall but allowed to remain within the peritoneal cavity. These trocars, along with the umbilical trocar, are used to close the small gastrotomies. Typically this is performed with figure-of-eight sutures, although an endoscopic linear stapler may be used.
An additional technique for transgastric cyst gastrostomy is to perform it intraperitoneally through an anterior gastrotomy. For this procedure, the patient is again placed in split leg or low lithotomy position. The surgeon stands between the legs and four trocars are typically required. A 5 to 10 mm camera port is placed at the umbilicus for the introduction of an angled (30° or 45°) laparoscope. A 5-mm working port is placed in a subxiphoid area and 5 to 12 mm ports are placed in the left and right subcostal areas.
After insufflation of the abdomen, the anterior gastric wall over the bulge of the pseudocyst is opened, using scissors with cautery, ultrasonic dissection, or an endoscopic linear stapler. Following the gastrotomy, the pseudocyst is localized by vision, palpation, or the insertion of a 22-gauge spinal needle. The posterior gastric wall is then opened into the pseudocyst, again using one of the various energy sources or linear-cutting staplers, and the fluid from the pseudocyst is fully aspirated. The laparoscope can be moved to the epigastric port for direct visualization within the pseudocyst itself. Any necrotic material within the pseudocyst is removed and placed into a laparoscopic impermeable bag.
The pseudocyst is secured to the gastric walls using either a laparoscopic linear stapler or permanent or absorbable sutures. Our preference is to suture the cyst gastrostomy to reduce as much as possible the incidence of bleeding.
At the completion of the cyst gastrostomy, a nasogastric tube is inserted into the stomach and placed in the cyst gastrostomy. The anterior gastric wall can be closed using a linear cutting stapler or sutures.
Laparoscopic Pancreatic Cyst Jejunostomy.
Abdominal access is gained through the umbilicus in the usual fashion. Typically, two trocars are placed lateral and superior to the umbilicus on the left. One trocar is similarly placed just outside the rectus muscle on the patient’s right. The most lateral left trocar typically is a 12-mm port, which will permit the use of a GI stapler. After general inspection of the abdomen, the omentum and transverse colon are rolled upward. Frequently the patient will need to be placed in steep Trendelenburg position to assist with this maneuver. Most often, the pancreatic pseudocyst can be visualized through the transverse mesocolon. If there is any doubt of its location, laparoscopic ultrasound can easily visualize the fluid collection through the mesocolon. Just superior and lateral to the ligament of Treitz is the preferred site of entry into the pseudocyst. Aspiration with a laparoscopic decompression needle can be performed to further ensure the pseudocyst location.
With the transverse colon retracted as far superiorly as possible, the jejunum is measured approximately 30 cm distal to the ligament of Treitz and transected. Before transecting it, we ensure that it reaches easily up to where we intend to open the pseudocyst. Using ultrasonic dissection, the pseudocyst is then opened through the transverse mesocolon. The initial window is quite small and the laparoscopic suction device is used to limit flow of the pseudocyst fluid into the abdominal cavity. After opening the window slightly larger, the laparoscope can actually be placed into the pseudocyst for full visualization. Any necrotic debris can be removed through this window. A small enterotomy is then made in the Roux limb and a stapled cyst jejunostomy can be performed. The staple line is inspected very closely, and any oozing points are managed with interrupted figure-of-eight sutures placed intracorporeally. The cyst enterostomy is then closed using running 2-0 silk. The procedure is completed with the jejunojejunostomy performed at least 30 cm distal to the cyst jejunostomy. The abdomen is irrigated profusely to remove any and all pancreatic debris and fluid that leaked during the operation.
Laparoscopic Distal Pancreatectomy
Patient position and trocar placement for LDP are similar to those described previously for cyst gastrostomy via the lesser sac approach. 4 The splenic flexure of the colon is mobilized and the gastrocolic omentum is divided (Fig. 4), preserving the gastroepiploic artery. The lesser sac is entered and exposure of the pancreas is enhanced by grasping the posterior gastric wall and elevating it cephalad (Fig. 5). To preserve the spleen, the tail of pancreas must be disconnected from it by means of meticulous dissection, the application of hemoclips, and the judicious use of an ultrasonic dissector. Arterial and venous branches to the pancreas must be painstakingly ligated and divided to avoid major hemorrhage. Mobilization of the pancreas is continued medially, usually to the point of the inferior mesenteric vein inflow to the splenic vein (Fig. 6). The peritoneum overlying the inferior border of the pancreas is incised, and via this largely avascular plane of dissection the lower border of the pancreas can be elevated, allowing for retroperitoneal pancreatic access. It is often necessary to use this access to separate the splenic vein from the pancreatic parenchyma in the process of the spleen-preserving distal pancreatectomy. A linear stapling device is then placed across the pancreas at the selected resection line. Once the stapler is fired, the surgeon may choose to oversew the staple line as an added measure to ensure pancreatic ductal closure (Fig. 7). We do so routinely.
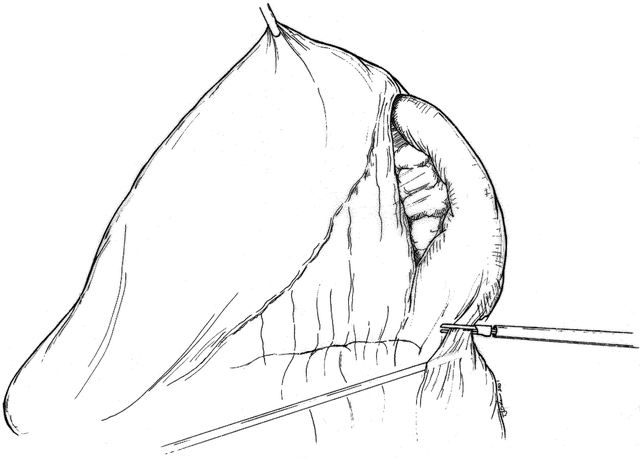
Figure 4. Mobilization of splenic flexure of colon and entry into lesser sac.
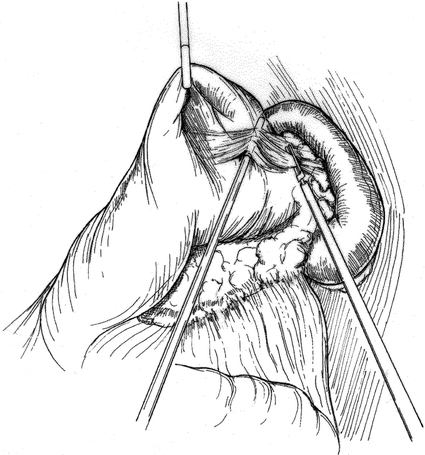
Figure 5. Retraction of stomach, exposing tail of the pancreas.
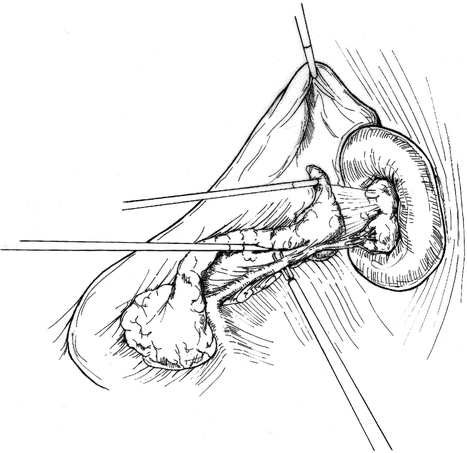
Figure 6. The tip of the pancreas is mobilized. Small branching arteries and veins are divided. The splenic vein is dissected off the pancreas.
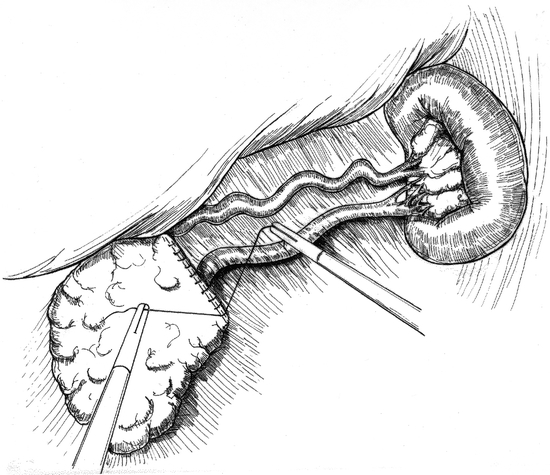
Figure 7. Stapled edge of remaining pancreas is oversewn. Splenic artery and vein are preserved, as is the spleen.
The technique of LDP with en bloc splenectomy is fairly straightforward. Once again the splenic flexure of the colon must be mobilized and the gastrocolic omentum divided. Tilting the operating room table so that the patient’s left side is elevated 30° to 45° may facilitate mobilization of the spleen. All attachments of the spleen are divided, including the short gastric vessels. The peritoneum over the inferior border of the pancreas is incised. It is often helpful to ligate and divide the proximal splenic artery early in the procedure (Fig. 8). If the splenic vein is easily accessed by posterior pancreatic dissection or superiorly on the pancreas, it should be isolated and divided. If, however, it is deeply embedded within the parenchyma of the pancreas, it may be divided as such using the linear stapling device. Whether or not splenic preservation is performed with LDP, a Jackson-Pratt drain is placed in the bed of the pancreatic dissection and drawn out through one of the 5-mm port sites. It is removed on day 1 or 2 postoperatively, once it has been determined that there is no gross pancreatic fluid leak.
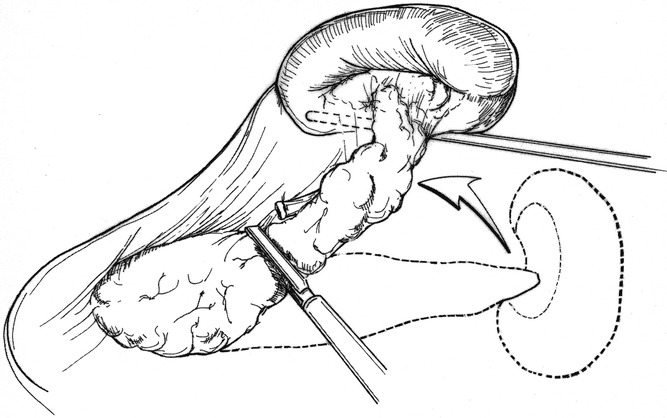
Figure 8. En bloc resection of the tail of the pancreas and spleen. The entire specimen is rolled medially.
In two cases, hand-assisted laparoscopic pancreatic resections were performed. In both cases patient set-up and trocar positioning were essentially similar to that used for LDP. The difference was that a hand access device was placed through the necessary incision in the right upper quadrant. This allowed introduction of the surgeon’s left hand. In one case a distal pancreatectomy was performed, and in the other an insulinoma was palpated in the neck of the pancreas and enucleated.
RESULTS
Laparoscopic Treatment of Pancreatic Pseudocysts
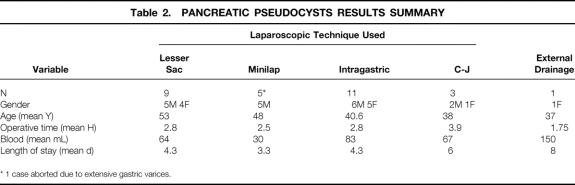
Laparoscopic treatment of PP was attempted by means of four distinct techniques in 29 patients (18 men, 11 women) with a mean age of 45 years (range 25–78) and completed successfully in 28. (In one patient in the minilaparoscopic cyst gastrostomy group, extensive gastric varices were encountered and the procedure was aborted.) The overall mean operative time was 2.8 hours and the mean postoperative length of stay (LOS) was 4.4 days (range 2–8). Perioperative data are summarized in Table 2 for each of the techniques used in this study.
Table 2. PANCREATIC PSEUDOCYSTS RESULTS SUMMARY
* 1 case aborted due to extensive gastric varices.
Laparoscopic cyst jejunostomy was performed on only three patients. Mean operative time was 3.9 hours, and postoperative LOS averaged 6 days. The laparoscopic cyst jejunostomy was the most technically challenging of the procedures used in this study for the treatment of PP and appeared to confer little benefit to the patients in terms of postoperative recovery. Early experience with intragastric cyst gastrostomy resulted in a mean operative time of 2.8 hours and a mean postoperative LOS of 4.3 days. Although there were no intraoperative bleeding complications, two patients in this group did bleed postoperatively, requiring blood transfusions. The bleeding did not necessitate further surgery and most likely arose from the anastomosis. In subsequent patients undergoing intragastric cyst gastrostomy the anastomosis was sutured to ensure hemostasis. This was a very technically demanding procedure. The lesser sac approach to laparoscopic cyst gastrostomy was developed in part as a result.
The two techniques of laparoscopic cyst gastrostomy that we now most commonly use are the minilaparoscopic cyst gastrostomy and the cyst gastrostomy by the lesser sac approach.
Operative times for the minilaparoscopic cyst gastrostomy averaged 2.5 hours, and the average amount of fluid removed from the pseudocyst was 2,167 mL. A postoperative CT scan was performed in each case within 1 week of surgery, revealing minimal fluid remaining within the cyst cavity. Postoperative LOS averaged 3.3 days, and all patients were discharged home in stable condition, tolerating a regular diet. With a mean follow-up of 15.8 months (range 1–36), the patients remained asymptomatic without recurrence of their PP.
Nine patients (5 men, 4 women) ranging in age from 34 to 78 years (mean 53 years) underwent laparoscopic cyst gastrostomy by a lesser sac approach. Mean operative time in this group was 2.8 hours and mean blood loss was 64 mL. These patients’ average postoperative LOS was 4.3 days; no intraoperative or postoperative bleeding complications occurred in this group. However, one elderly woman with multiple medical problems suffered a myocardial infarction and died 1.5 months postoperatively. She had had an uneventful postoperative course and had been discharged home stable and tolerating a regular diet on postoperative day 4. Also, a 44-year-old man whose pseudocyst arose secondary to alcoholic pancreatitis returned to the hospital with acute pancreatitis 3 weeks following his surgery. He had also had an uneventful surgery and postoperative course to that point. This patient subsequently developed necrotizing pancreatitis, and although he survived the process he did require multiple open debridement procedures on his remaining pancreas.
A 47-year-old woman with an expanding and increasingly symptomatic pseudocyst that arose during an episode of acute pancreatitis underwent a diagnostic laparoscopy. It was determined at that time that the pseudocyst was not amenable to internal drainage. A cystotomy was made through which the contents of the cyst were evacuated and a laparoscopic pancreatic necrosectomy was performed. A mediastinal sump drain that was initially used for irrigation and drainage was then inserted directly into the cyst cavity though the cystotomy. The patient also underwent endoscopic placement of a transpapillary stent. Her pancreatic drainage declined steadily postoperatively and she was released home on postoperative day 8.
Laparoscopic Distal Pancreatectomy
Laparoscopic distal pancreatectomy was attempted in 25 patients (9 men, 16 women) ranging in age from 36 to 73 years (mean 48.9 years) who presented consecutively with lesions in their distal pancreas (or neck); underlying pathology is summarized in Table 1. Distal or subtotal laparoscopic pancreatectomy was completed successfully in 23 patients. Two patients underwent a successful hand-assisted laparoscopic enucleation of an insulinoma. Mean operating time was 3.7 hours (range 1.8 to 7 hours) Intraoperative blood loss ranged from 30 to 1200 mL (mean 273.9mL) and the mean length of stay was 4.1 days (range 2 to 8 days).
All patients in whom insulinomas were diagnosed underwent a progression of least to more invasive imaging and localizing studies. A CT scan of the abdomen was initially obtained, followed, if indicated, by MRI. An octreotide scan was performed and was negative in four insulinoma patients. In the same patients endoscopic ultrasound was also used to no avail in a further attempt to localize the lesions. Selective arterial calcium injection with hepatic venous sampling was performed in the four patients in whom the insulinomas remained elusive. In two of these patients venous sampling studies strongly suggested tumors of the pancreatic tail; this was borne out at laparoscopy. In another patient (a 73-year-old woman) preoperative venous sampling indicated a pancreatic head/neck location for the insulinoma. This was localized and enucleated as noted above. The fourth patient, a 46-year-old woman, also underwent preoperative venous sampling that weakly suggested the possibility of a lesion of the head or neck of the pancreas. This patient underwent diagnostic laparoscopic and laparoscopic ultrasound, still without identification of the tumor. Finally, when a laparotomy was performed the insulinoma was palpated in the head of the pancreas and was enucleated in a standard fashion. A second case, involving a 58-year old man with chronic pancreatitis (alcohol related) and a cyst in the tail of his pancreas, underwent conversion to laparotomy. Laparoscopic exploration was attempted but proved fruitless. There were extensive inflammatory changes along the body and tail of the pancreas and essentially obstruction of the lesser sac. The decision to convert to laparotomy was soon made. The patient subsequently underwent open distal pancreatectomy, splenectomy and partial gastrectomy with an operating time of 5 hours. He recovered uneventfully and was discharged home on the seventh post-operative day.
There were four complications among the LDP cases. These included two cases of intraoperative bleeding requiring blood transfusion, one wound infection, and one pancreatic duct leak. Both cases of intraoperative bleeding were in patients who had insulinomas. In one (obese) patient who underwent hand-assisted distal pancreatectomy, a wound infection developed in the hand port site. Despite oversewing of the stapled end of the pancreatic remnant, one pancreatic duct leak occurred, presenting as a post-operative pseudocyst and drained percutaneously.
Twelve patients in this series (48%) underwent successful removal of their pancreatic tumors with preservation of the spleen. In three (43%) of the seven patients with insulinoma who underwent successful LDP it was necessary to resect the spleen en bloc.
DISCUSSION
Pancreatic Pseudocysts
The management of PPs has undergone much change over the past decade. 8–12 Minimally invasive therapies for the treatment of PP have generated interest recently, but alternative approaches have also been explored. One alternative is percutaneous CT- or ultrasound-guided drainage of the PP. 13 Although there is low mortality associated with this procedure, its morbidity remains fairly high, with a failure rate of up to 70%. 14 Complications of this procedure include cyst recurrence, bleeding, infection, pancreatic fistula, and drain tract infections. 13,14 Failed percutaneous drainage leads to prolonged hospitalization, increased cost, morbidity, and delay until definitive drainage can be achieved. 15 However, percutaneous drainage does serve a role for the treatment of PP in cases of immature or infected cysts, in the pediatric population, and in patients with prohibitively high surgical risk. 16
Endoscopic internal procedures have become increasingly popular and prevalent. 17 Whereas reports of percutaneous radiologic drainage of PP date back more than a decade, the experience with endoscopic PP treatment has developed in parallel with other laparoscopic procedures over the past 5 or 6 years. 18,19 Although reported success rates range from 65% to 100% in selected patients, 9,20 endoscopic internal drainage procedures risk major complications, including bleeding and perforation. 21 In addition, there are significant technical limitations for endoscopic drainage procedures. Cyst/enteric anastomoses are usually limited to 1 to 1.5 cm in size. The incidence of bleeding complications increases with increasing size of the anastomoses. To avoid this complication, stent placement with various catheters has been utilized. Stents ranging from 7F to 12F have been used singly or by placing two stents via one cyst gastrostomy. However, common stent problems include dislodgment, kinking, perforation, and clogging with viscous cyst fluid and tissue debris. 20 Inadequate drainage of necrotic debris may lead to cyst infection. 22 Moreover, multiple pseudocysts are present in 18.5% of these patients, 23 and although multiple procedures can be carried out in a single patient, technical limitations can lead to inadequate drainage.
A variety of laparoscopic approaches to pseudocyst management have been appearing in the literature. One of the most reported techniques has been to perform a gastrotomy laparoscopically followed by a cyst gastrostomy via the posterior gastric wall. This has been an effective means of drainage and can be performed with a stapler or intracorporeal suturing techniques. It does require a generous anterior gastrotomy, which must be closed. The advantage of the “lesser sac approach” described earlier is avoidance of the anterior gastrotomy while preserving the benefit of the generous, stapled anastomosis. On occasion, however, inflammation may result in obliteration of the lesser sac, precluding completion of the pseudocyst gastrotomy via the lesser sac approach.
Most recently, combining the procedures of upper endoscopy and intragastric, laparoscopic surgery to create pancreatic cyst gastrostomies has been described. 18,24–26 The latest modifications of these evolving techniques utilize standard laparoscopic instruments placed through balloon ports into the gastric lumen. The use of 1.7 to 2 mm minilaparoscopic instruments that pass through small-gauge ports obviates the need for pneumoperitoneum and standard laparoscopic instruments for this procedure. In our limited series, this technique was effective with no morbidity, early discharge from the hospital, and no recurrence of the pseudocyst.
One patient in our series underwent laparoscopic drainage of the PP, including necrosectomy and subsequent placement of a sump drain through the cystotomy. Although this is the least common of the reported laparoscopic PP procedures, it has been previously described. 27 More recently, Oria et al reported the technique of video-assisted pancreatic necrosectomy, 28 using a laparoscope inserted through a limited pseudocystotomy at the time of laparotomy for the internal drainage of giant acute pseudocysts. Clearly adequate numbers and long-term follow-up data are lacking, but there are some proposed advantages to various techniques of laparoscopic external drainage of PPs. They include the avoidance of retroperitoneal sepsis and hemorrhage by adequate, visually directed drainage and debridement of cyst contents;28 and the avoidance of potential injury to dilated collateral veins or adjacent gastrointestinal organs at risk during ultrasound- or CT-guided PP drainage.
Laparoscopic Distal Pancreatectomy
The history of minimally invasive resection of the pancreas is a brief one. Gagner and Pomp 29 reported their initial experience with laparoscopic resections of the pancreas extending back to early 1992, and since then others have reported their experience in a variety of settings. 17,30–32 Although clinical experience with these procedures remains limited, an early consensus appears to have emerged. Several authors have noted that LDP offers patient benefits in terms of postoperative recovery and LOS, coupled with acceptable perioperative morbidity and complication rates. Most of these authors state their intent to explore this procedure further and laud its potential.
Conversely, very limited experience reported with laparoscopic pancreaticoduodenectomy suggests that it is not a procedure that confers any patient benefit when compared to the open surgery and should not be considered nor offered to patients 4,5 at present.
The first accounts of distal pancreatectomy date back to the late 19th century and early 20th century. 33,34 Through much of the 20th century this was a relatively rare procedure associated with significant perioperative morbidity and mortality. 35,36 It has been only over the past decade or so that significant improvements in operative outcomes of conventional distal pancreatectomy have been reported 35,37 as experience with the procedure has grown in specialized centers.
In the largest series of distal pancreatectomies published to date, Lillimoe et al. reported their experience with 235 cases performed at a single institution over a 14-year period. 35 Their perioperative mortality rate was 0.9%, and the overall complication rate was 31%. However, included in that number was an overall 8% rate of development of new-onset diabetes mellitus, which others have suggested should more correctly be labeled as a predictable outcome of the procedure. 35 Mean operative time in this series was 4.7 hours (median 4.3 hours), mean blood loss was 879 mL (median 450 mL), and mean LOS was reportedly 15 days (median 10 days). Fernandez-del Castillo et al. reported their experience with 71 cases of conventional distal pancreatectomy in which they observed a perioperative mortality rate of 1.4% and a 20% incidence of complications. 37
By comparison, our early results with LDP are encouraging. In 25 cases (23 completed laparoscopically) there was no perioperative mortality, and the complication rate was 16%. Our mean operative time was 3.7 hours, mean blood loss was 270 mL, and mean postoperative LOS was 4.1 days.
Our experience also demonstrates the feasibility of employing a spleen-preserving technique when performing LDP. Several authors have recently described their experience with spleen-preserving (open) distal pancreatectomy. 38–40 The advantages to the patient, including eliminating the risk of overwhelming postsplenectomy sepsis, are obvious.
It may be argued that preservation of the spleen can be accomplished by preserving the short gastric (SG) vessels while sacrificing the splenic artery and vein during distal pancreatectomy. Certainly such an approach would be technically easier than painstakingly separating the pancreas from intact splenic vessels. However to achieve laparoscopic access and adequate exposure of the distal pancreas, several of the SG vessels must be divided which, combined with a divided splenic pedicle, would likely render the spleen nonviable.
Forty-eight percent of our LDP patients retained their spleens. Splenic preservation was attempted in all patients who were observed before and during surgery to have benign pancreatic pathology. Of note, the two cases in which the greatest intraoperative blood loss was recorded (1,200 mL and 800 mL) both had insulinomas that would not separate from their splenic veins. Ultimately, in both cases, after prolonged operative time and blood loss as noted, the spleen was resected en bloc with the pancreas. It has been our experience that when an insulinoma is in close proximity to the splenic vein a fairly intense desmoplastic reaction is often present, precluding preservation of the spleen. Otherwise the magnification and enhanced laparoscopic visualization of the operative field facilitates dissection, ligation, and division of branching arteries and veins into the pancreas.
As demonstrated by our experience with two cases in this series in which extensive preoperative and even intraoperative studies (including intraoperative ultrasound) failed to identify the insulinoma, there remains no minimally invasive substitute for manual palpation and exploration of the pancreas, particularly the head and neck. Recent advances in hand-assisted laparoscopic surgery have helped address this limitation of purely laparoscopic surgery. The drawback of hand-assisted laparoscopic surgery, as we found in this study, is that often a larger-than-anticipated incision for the surgeon’s hand is required; this carries with it the attendant risk of wound complications.
Therapeutic laparoscopy of the pancreas is still correctly described as experimental surgery by many surgeons. Many issues remain to be clarified in determining the future of these new procedures. Which patient should be selected? Which condition should be treated? Which procedures should be performed laparoscopically? Should patients with known or suspected pancreatic malignancy be offered this surgery? It is unlikely, other than in a very select and limited multicenter study, that the ideal randomized perspective trial(s) needed to answer these questions could be undertaken. The appropriate pancreas cases are too few and pathologies too diverse. Institutes with advanced laparoscopic expertise sufficient to perform these procedures rarely coexist with centers where adequate volumes of such pancreatic cases are seen. It may prove to be more feasible to orchestrate a prospective randomized trial comparing minimally invasive with endoscopic treatments of PPs. Patient accrual to such a trial may be less problematic than with other studies (e.g., laparoscopic versus open splenectomy [personal experience]) where patients will not submit to the possibility of randomization to open surgery.
CONCLUSIONS
This is to our knowledge the largest series of therapeutic laparoscopic pancreatic cases reported to date. It is not possible, however, to draw from this study any definitive conclusions regarding the advantages or otherwise of the laparoscopic approach, nor which patients or pancreatic conditions are best suited to these techniques. This series does, however, serve to demonstrate, as did the early series of laparoscopic fundoplication or laparoscopic splenectomy, that these new laparoscopic procedures are feasible and practical and that the results are reproducible. Our experience suggests that patients with pancreatic pseudocysts that are large or not amenable (anatomically or technically) to endoscopic drainage should be considered candidates for a laparoscopic internal drainage procedure. As well, patients with neuroendocrine pancreatic tumors and cystic or other (presumed) benign masses in the body and tail of the pancreas may benefit from laparoscopic extirpation of such lesions. Laparoscopic distal pancreatectomy, pseudocyst surgery, and enucleation of insulinomas performed in selected patients can result in a rapid postoperative recuperation with no mortality and few complications.
Footnotes
Correspondence: Adrian E. Park, MD, FRCSC, FACS, Center for MIS, Department of Surgery, University of Kentucky Chandler Medical Center, 800 Rose Street, Lexington, KY 40536-0293.
E-mail: apark@uky.edu
Supported in part by an educational grant from Tyco/U.S. Surgical Corporation.
Accepted for publication February 18, 2002.
References
- 1.Kelling G. Ueber oesophagoskopie, gastroskopie und kölioskopie. Münch Med Wochenschr 1902; 49: 21–24. [Google Scholar]
- 2.Jacobaeus HD. Über laparo- und thorakoskopie. Beitr Klin Tuberk 1912; 25: 185–354. [Google Scholar]
- 3.Meyer-Burg J, Ziegler U, Palma G. Zur supragastralen pankenskopic. Ergebnisse aus 125 laparoskopien. Dtsch Med Wochenschr 1973; 97: 1969–1971. [DOI] [PubMed] [Google Scholar]
- 4.Park A, Schwartz R. Laparoscopic pancreatic surgery. Am J Surg 1999; 177: 158–163. [DOI] [PubMed] [Google Scholar]
- 5.Ishida H. Peritoneoscopy and pancreas biopsy in the diagnosis of pancreatic disease. Gastrointes Endoscop 1983; 29: 211–218. [DOI] [PubMed] [Google Scholar]
- 6.Cuschieri A. Laparoscopic surgery of the pancreas. J Roy Coll Surg Edinb 1994; 39: 178–184. [PubMed] [Google Scholar]
- 7.Gagner M, Pomp A, Herrera MF. Early experience with laparoscopic resections of islet cell tumors. Surgery 1996; 120: 1051–1054. [DOI] [PubMed] [Google Scholar]
- 8.Adams DB, Anderson MC. Changing concepts in the surgical management of pancreatic pseudocysts. Am Surg 1992; 58: 173–180. [PubMed] [Google Scholar]
- 9.Lawson JM, Biallie J. Endoscopic therapy for pancreatic pseudocysts. Gastrointest Clin North Am 1995; 5: 181–193. [PubMed] [Google Scholar]
- 10.Moran B, Rew DA, Johnson CD. Pancreatic pseudocysts should be treated by surgical drainage. Ann Roy Coll Surg Engl 1994; 76: 54–58. [PMC free article] [PubMed] [Google Scholar]
- 11.Sanfey H, Aguilar M, Jones RS. Pseudocysts of the pancreas, a review of 97 cases. Am Surg 1994; 60: 661–668. [PubMed] [Google Scholar]
- 12.Vitas GJ, Sarr MG. Selected management of pancreatic pseudocysts: operative versus expectant management. Surgery 1992; 111: 123–130. [PubMed] [Google Scholar]
- 13.Adams DB, Anderson MC. Percutaneous catheter drainage compared with internal drainage in the management of pancreatic pseudocyst. Ann Surg 1992; 215: 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Criado E, DeStefano AA, Weiner TM, et al. Long-term results of percutaneous catheter drainage of pancreatic pseudocysts. Surg Gynecol Obstet 1992; 175: 293–298. [PubMed] [Google Scholar]
- 15.Rao R, Fedorak I, Prinz RA. Pancreatic pseudocyst should be treated by surgical drainage. Ann Roy Coll Surg Engl 1994; 76: 54–58. [PMC free article] [PubMed] [Google Scholar]
- 16.Balthazar EJ, Freeney PC, van Sonnenberg E. Imaging and intervention in acute pancreatitis. Radiology 1994; 193: 297–306. [DOI] [PubMed] [Google Scholar]
- 17.Grace PA, Williamson RC. Modern management of pancreatic pseudocysts. Br J Surg 1993; 80: 573–581. [DOI] [PubMed] [Google Scholar]
- 18.Targarona EM, Pera M, Martinez J, et al. Laparoscopic treatment of pancreatic disorders: diagnosis and staging, palliation of cancer and treatment of pancreatic pseudocysts. Int Surg 1996; 81: 1–5. [PubMed] [Google Scholar]
- 19.Way LW, Legha P, Mori T. Laparoscopic pancreatic cystogastrostomy: the first operation in the new field of intraluminal laparoscopic surgery. Surg Endosc 1994; 8: 235–239. [Google Scholar]
- 20.Smits ME, Rauws EA, Tytgat GN, et al. The efficacy of endoscopic treatment of pancreatic pseudocysts. Gastrointest Endoscop 1995; 42: 202–207. [DOI] [PubMed] [Google Scholar]
- 21.DeGuzman KP, Holderman WH, Abu-Hamour A, et al. Endoscopic drainage of pancreatic pseudocysts: patient selection and evaluation of the outcome by endoscopic ultrasonography. Endoscopy 1995; 27: 329–333. [DOI] [PubMed] [Google Scholar]
- 22.Hariri M, Slivka A, Carr-Locke DL, et al. Pseudocyst drainage predisposes to infection when pancreatic necrosis is unrecognized. Am J Gastroenterol 1994; 89: 1781–1784. [PubMed] [Google Scholar]
- 23.Fedorak IJ, Prinz RA. The clinical challenge of multiple pancreatic pseudocysts. Am J Surg 1994; 168: 22–28. [DOI] [PubMed] [Google Scholar]
- 24.Atabek U, Mayer D, Amin A, et al. Pancreatic cystogastrostomy by combined upper endoscopy and percutaneous transgastric instrumentation. J Laparoendosc Surg 1993; 3: 501–504. [DOI] [PubMed] [Google Scholar]
- 25.Gagner M. Laparoscopic transgastric cystogastrostomy for pancreatic pseudocyst [abstract]. Surg Endosc 1994; 8: 239. [Google Scholar]
- 26.Trìas M, Targarona EM, Balagué; C, et al. Intraluminal stapled laparoscopic cystogastrostomy for treatment of pancreatic pseudocyst. Br J Surg 1995; 82: 403. [DOI] [PubMed] [Google Scholar]
- 27.Takami Y, Tajima Y, Inoue K, et al. Laparoscopic external drainage of a pancreatic pseudocyst associated with periportal collaterals. Surg Laparosc Endosc 1998; 8: 241–244. [PubMed] [Google Scholar]
- 28.Oria A, Ocampo C, Zandalazini H, et al. Internal drainage of giant acute pseudocysts: the role of video-assisted pancreatic necrosectomy. Arch Surg 2000; 135: 136–141. [DOI] [PubMed] [Google Scholar]
- 29.Gagner M, Pomp A. Laparoscopic pancreatic resection: is it worthwhile? J Gastrointest Surg 1997; 1: 20–26. [DOI] [PubMed] [Google Scholar]
- 30.Cuschieri A, Jakimowicz JJ, van Spreeuwel J. Laparoscopic distal pancreatectomy procedures in a rural hospital. Ann Surg 1996; 223: 280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sussman LA, Christie R, Whittle DE. Laparoscopic excision of distal pancreas including insulinoma. Aust NZ J Surg 1996; 66: 414–416. [DOI] [PubMed] [Google Scholar]
- 32.Tihanyi TF, Morvay K, Nehez L, et al. Laparoscopic distal resection of the pancreas with the preservation of the spleen. Acta Chir Hung 1997; 36: 359–361. [PubMed] [Google Scholar]
- 33.Finney JMT. Resections of the pancreas. Am Surg Assoc 1910: 315–330. [Google Scholar]
- 34.Mayo WJ. The surgery of the pancreas. Ann Surg 1913; 58: 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lillemoe KD, Kaushal S, Cameron JL, et al. Distal pancreatectomy: Indications and outcomes in 235 patients. Ann Surg 1999; 229: 673–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gudjousson B. Cancer of the pancreas: 50 years of surgery. Cancer 1987; 60: 2284–2203. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-del Castillo C, Fattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg 1995; 130: 295–300. [DOI] [PubMed] [Google Scholar]
- 38.Aldridge MC, Williamson RCN. Distal pancreatectomy with and without splenectomy. Br J Surg 1991; 78: 976–979. [DOI] [PubMed] [Google Scholar]
- 39.Richardson DQ, Scott-Conner CEH. Distal pancreatectomy with and without splenectomy. Am Surg 1989; 55: 21–25. [PubMed] [Google Scholar]
- 40.Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg 1988; 123: 550–553. [DOI] [PubMed] [Google Scholar]