Abstract
Objective
To determine the feasibility of surgically correcting pectus excavatum and carinatum deformities in adult patients.
Summary Background Data
Although pectus chest deformities are common, many patients progress to adulthood without surgical repair and experience increasing symptoms. There are sparse published data regarding repair of pectus deformities in adults.
Methods
Since 1987, 116 patients over the age of 18 years with pectus excavatum (n = 104) or carinatum (n = 12) deformities underwent correction using a highly modified Ravitch repair, with a temporary internal support bar. The ages ranged from 19 to 53 years (mean 30.1). Eighty-six patients sought repair after reviewing information regarding pectus deformities available on the Internet. Each patient experienced dyspnea with mild exertion and decreased endurance; 84 had chest pain with activity; 75 had palpitations and/or tachycardia. Seven patients underwent repair for symptomatic recurrent deformities. The mean severity score (chest width divided by distance from sternum to spine) was 4.8. The sternal bar was removed from 101 patients 6 months after the repair without complications.
Results
Each of the patients with reduced endurance or dyspnea with mild exercise experienced marked improvement within 6 months. Chest discomfort was reduced in 82 of the 84 patients. Complications included pleural effusion (n = 7), pneumothorax (n = 2), pericarditis (n = 2), dislodged sternal bar (n = 3), and mildly hypertrophic scar (n = 12). Mean hospitalization was 2.9 days; mean blood loss was 122 mL. Pain was mild and of short duration (intravenous analgesics were used a mean of 2.1 days). There were no deaths. With a mean follow-up of 4.3 years, 109 of 113 respondents had a very good or excellent result.
Conclusions
Although technically more difficult than in children, pectus deformities may be repaired in adults with low morbidity, short hospital stay, and very good physiologic and cosmetic results.
Pectus excavatum (PE) is by far the most common major congenital chest deformity, occurring in approximately 1 in every 400 births (personal communication, March of Dimes Birth Defects Foundation, March 1995). 1 Although the majority of patients with PE are recognized during the first year of life, the depression usually becomes much more severe during the period of rapid skeletal growth in early adolescence. In contrast, pectus carinatum (PC) deformities are often unrecognized until adolescent skeletal growth occurs. 2 The majority of PE and PC deformities will remain with the same severity throughout adult life after full growth has been achieved. With PE, the heart is often displaced into the left chest by the depressed sternum, and pulmonary expansion is confined. With PC, the thorax is held in a partially expanded position, with increased residual air and reduced vital capacity. The recognition of symptoms and the recommendation for surgical correction remain controversial, with strong divergent opinions being expressed regarding whether PE and PC are primarily cosmetic disorders, or if they cause physiologic impairment and limitations to the patient. This controversy has been accentuated in recent years as hospital charges have increased and insurance coverage for many medical conditions has been more restricted.
Compounding the decision of whether to repair pectus deformities is the lack of reliable and consistent routine cardiopulmonary function studies that clearly indicate the physiologic limitations on a specific patient, or the improvement that occurs after operation. 3 Until the past 5 years, few hospitals had compiled a large surgical experience, with most surgeons performing only a few pectus operations each year using a variety of surgical techniques. The reported results have been inconsistent, further confusing the decision of whether to correct the deformity. A large number of patients have therefore remained with uncorrected deformities as they have progressed through adulthood.
During the past 5 years, with the revolutionary new concept of minimally invasive PE repair (MIRPE) reported by Nuss et al. 4 and the availability of several informative pectus websites on the Internet, many patients of all ages have become aware that their deformities can be corrected with a high success rate. Although the majority of pectus repairs have been performed on young children and adolescents, an increasing number of patients 19 years and older have sought advice regarding repair of their uncorrected deformities because of persistent and often worsening symptoms. Although most children undergoing pectus repair are operated on by pediatric surgeons, adults seeking repair often consult adult thoracic surgeons, who frequently have limited experience correcting these deformities.
There is sparse published information regarding the repair of PE and PC deformities in adults. Following the report in 1999 of our initial surgical experience with 25 patients 20 years of age or older, 5 the present study provides a more comprehensive review of our experience with 116 PE and PC patients 19 years of age or older who have undergone surgical repair. Consent for the study was granted by the UCLA Medical Center Institutional Review Board.
METHODS
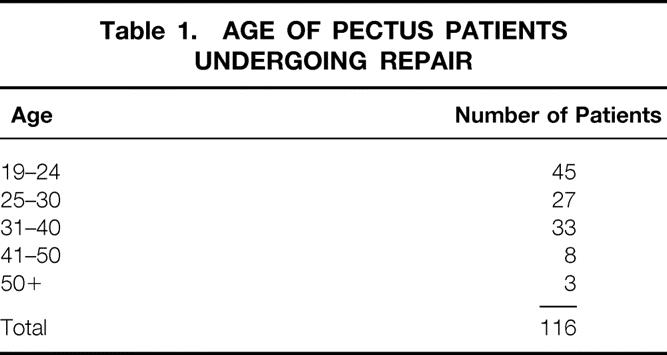
The medical records of all patients 19 years of age and older who underwent correction of PE and PC deformities at UCLA Medical Center from January 1969 through December 2001 were reviewed. During this 32-year period, 545 patients underwent repair of PE and 99 had correction of PC deformities. During the last 15 years, 116 of the patients were repaired at 19 years of age or older (18%), 106 during the past 6 years. Eighty-six patients sought repair after reviewing information regarding pectus deformities available on the Internet. The ages ranged from 19 to 53 years (mean 30.1 years) (Table 1). Twenty-four of the 116 were female (21%). One hundred four patients had PE and 12 had PC deformities; 3 PC patients had an associated mild PE of the lower chest. The deformity was asymmetric in 46 of the PE patients (44%) and 7 of the PC patients (58%). All but two of the operations were performed by one surgeon. The PE depression was evident within the first few months of life in 98 patients but was observed after the age of 10 years in each of the 12 PC patients. A family history of pectus deformities was identified in 32% of patients. Seven patients were referred for repair of symptomatic recurrent PE deformities.
Table 1. AGE OF PECTUS PATIENTS UNDERGOING REPAIR
The most frequent symptoms, which in varying degree were common to all of the patients, were dyspnea with mild exercise and progressive loss of stamina and endurance with physical activity. Many were able to participate in short-duration competitive athletic activities during their adolescent years but found it increasingly difficult to keep up with peers as they progressed through adulthood. Occasional compression-type discomfort in the lower anterior chest with activity was noted by 84 PE patients (81%). Palpitations and/or tachycardia were experienced by 73%. Exercise-induced wheezing was reported by 29%; 12% had asthmatic symptoms requiring bronchodilator medications. Eighteen percent had an increased frequency of respiratory infections compared to other persons their age, and often developed progression to a chronic cough. Twelve patients experienced occasional moderate to severe discomfort in the mid-posterior chest when swallowing solid foods; two had symptoms of mild gastroesophageal reflux. Functional heart murmurs were present in 22%; only two patients had congenital heart defects. Three patients had mild to moderate angina. Two patients had Marfan’s syndrome. Mild to moderate scoliosis of the thoracic spine was observed on chest roentgenograms of 81%; however, only one patient required specific therapy. None of the patients had other major medical disorders such as cancer, previous coronary artery occlusion, stroke, or chronic renal or pulmonary disease.
Electrocardiographic evidence of right ventricular strain was noted in 72% of PE patients; 18% had documented mitral valve prolapse. Twelve patients had a diastolic filling pattern suggesting constrictive dynamics. All 104 patients with PE had varying degrees of displacement of the heart into the left chest on radiographs and/or CT scans. The pectus severity index, calculated by dividing the inner width of the chest at the widest point by the distance between the posterior surface of the sternum and anterior surface of the spine, ranged from 3.2 to 9.3 (mean 4.8) for PE patients (4.49 for the 80 men and 5.94 for the 20 women). The mean severity index of normal adults is 2.5, and the mean index of patients who underwent PE repair in the large series reported by Haller et al. was 4.4. 6 The severity index for the PC patients ranged from 1.3 to 2.0 (mean 1.8). Chest computed tomography or magnetic resonance imaging scans were performed on 36 patients and showed narrow anterior-posterior chest diameters with compression and displacement of the heart in PE patients; an increase in the anterior-posterior diameter of the chest with narrow cardiac and mediastinal silhouette was noted in the PC patients.
The highly modified Ravitch repair (HMRR) operative technique used for each of the 116 patients was an extensive modification of that described by Ravitch 7 and Welch, 8 with less cartilage resection than reported previously, 9 and includes the following major features. General endotracheal anesthesia was used. An orogastric tube and Foley bladder catheter were placed for all patients. Intravenous cefazolin was given preoperatively. A transverse curvilinear incision was made midway between the nipples and costal margin, with a short midline extension superiorly in most patients (Fig. 1). Short skin flaps were elevated superiorly and inferiorly using needle-tip electrocautery. The pectoralis muscles were reflected laterally over a short distance, and the abdominal muscles were mobilized inferiorly sufficient to expose the deformed costal cartilages. Short cautery incisions (1.0–1.5 cm) were made through the perichondrium of deformed costal cartilages adjacent to the sternum; a second 1.0- to 1.5-cm incision was made laterally near or beyond the costochondral junction where the chest wall was at the highest level. Short segments of cartilage (1.0–1.5 cm) were resected medially and laterally from each of the deformed ribs using Freer and Haight elevators, carefully preserving the entire perichondrium. The xiphoid was detached from the lower sternum (Fig. 2). The lower two perichondrial sheaths were detached from the sternum bilaterally for all PE patients and for occasional PC patients. The remaining perichondrial sheaths remained attached to the sternum. The retrosternal space of PE patients was mobilized from 4 to 6 cm with cautery. The right pleural space was opened widely for drainage and a small chest tube was inserted. A transverse wedge osteotomy was made across the anterior table of the sternum at the level where the sternum angles to depress posteriorly (Fig. 3). The posterior table of the sternum was gently fractured at the osteotomy without detachment; the lower end of the sternum was then twisted when indicated and elevated to the desired position. For PC patients, a simple transverse osteotomy was made across the anterior sternum at the desired level when necessary and a wedge of autologous costal cartilage was placed into the osteotomy to depress the lower sternum to the desired level. The periosteum on each side of the osteotomy was loosely approximated with nonabsorbable sutures. A thin stainless-steel bar (Adkins strut) 10 (Baxter Health Care Corp. Operating Room Division, McGraw Park, IL) was placed obliquely across the lower anterior chest posterior to the sternum and through or posterior to the perichondrial sheaths to elevate the sternum as well as the anterolateral chest to the desired level (Fig. 4). The strut was attached to the appropriate rib on each side with fine wire. For PC patients, the sternal bar was placed external to the cartilaginous repair with a large absorbable suture placed through the sternum and attached to the sternal bar to provide stability. The xiphoid and perichondrial sheaths were sutured back to the lower sternum. Finely minced fragments of autologous costal cartilage, which had been removed earlier, were placed into the short segments of open perichondrial sheaths to enhance cartilage regeneration. The perichondrial sheaths were closed loosely. The pectoralis and abdominal muscles were sutured together over the sternal and costal cartilage repair. For PC patients, a small suction drainage catheter was placed between the muscle layer and the cartilaginous repair. Thorough hemostasis was achieved with electrocautery, and the wound was copiously irrigated with cefazolin solution throughout the operation. The skin was closed with absorbable subcuticular sutures and Steri-strips.
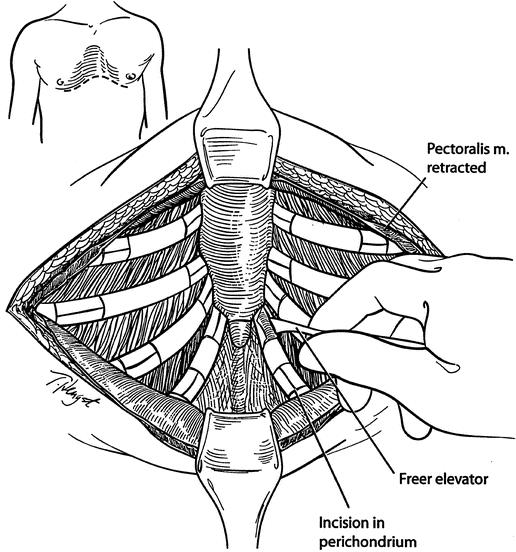
Figure 1. Pectoralis muscles are reflected laterally over a short distance, and abdominal muscles are reflected inferiorly to expose the deformed cartilages. Incisions are made through the perichondrium on the medial and lateral ends of the deformed cartilages, and short segments of cartilage are removed.
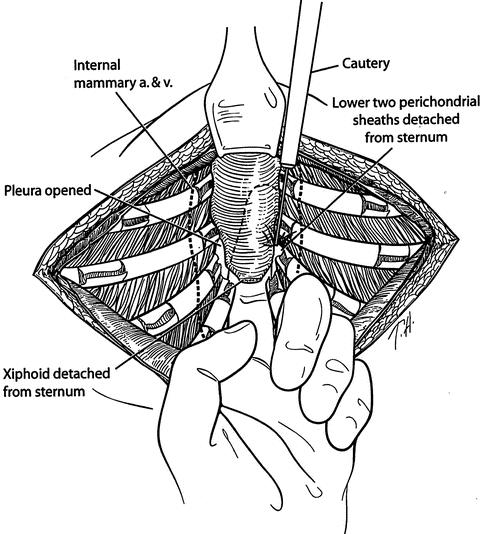
Figure 2. The xiphoid and lower two perichondrial sheaths are detached from the lower end of the sternum. The retrosternal space is mobilized using electrocautery. The right pleural space is opened for drainage. A chest tube is placed into the right pleural space.
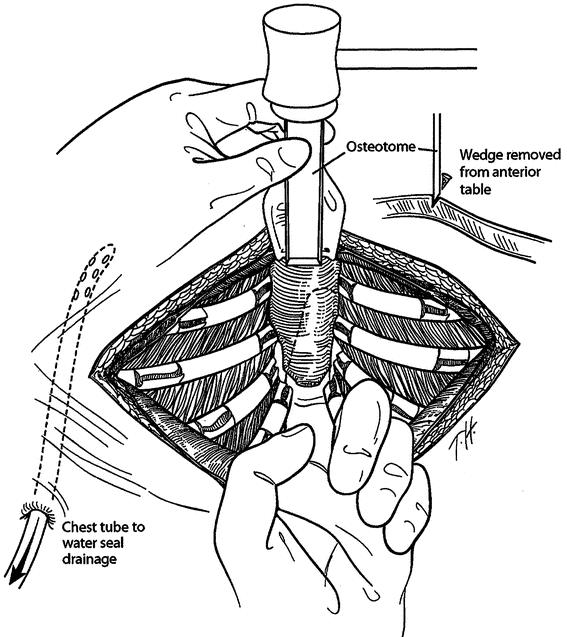
Figure 3. A transverse wedge osteotomy is made across the anterior table of the sternum at the desired level. The posterior table of the sternum is fractured but not detached; the lower sternum is elevated to the desired level. Sutures are placed across the osteotomy.
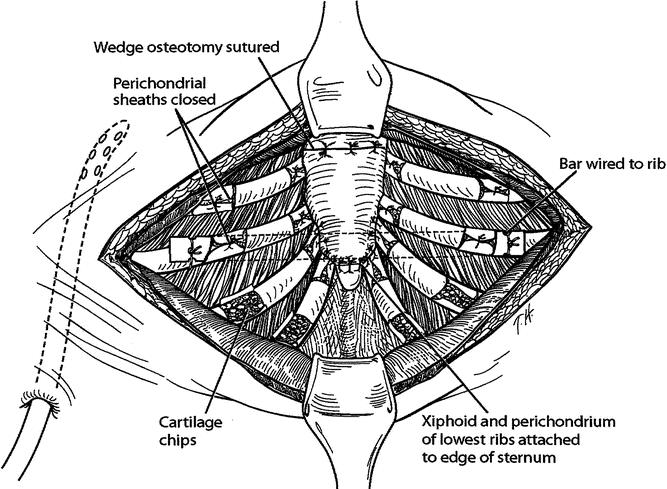
Figure 4. An Adkins strut is placed across the lower anterior chest posterior to the sternum and through or posterior to the perichondrial sheaths; the strut is attached to the anterior surface of a rib on each side with wire. The xiphoid and perichondrial sheaths are sutured back to the sternum. Finely minced fragments of autologous costal cartilage are placed into the open perichondrial sheaths. The perichondrial sheaths are closed loosely with sutures.
For 106 patients the endotracheal tube was removed in the operating room; the remaining endotracheal and all orogastric tubes were removed in the recovery room. No patients were placed in an intensive care unit. Postoperative care adhered to a clinical pathway program. The chest tube and Foley catheters were routinely removed within 24 hours. Intravenous cefazolin was given for 3 days, and oral cephalexin was given for 4 additional days. Postoperative pain was mild for almost all patients and was controlled with intravenous analgesics for the first 2 postoperative days, and by oral nonnarcotic medications thereafter. Epidural analgesia was not used for any patients.
The mean duration of the operation was 193 minutes (± 28), and 212 minutes (± 19) for patients undergoing repair of recurrent deformities. 11 A variety of modifications of the operative technique were used for patients with recurrent deformities, including the use of autologous rib grafts from the posterolateral chest. The mean blood loss was 122 mL; no patients received blood transfusions. None of the patients were hospitalized for more than 3 days (mean 2.9 days). All but seven patients returned to work or school within 2 weeks. The sternal support bar was removed on an outpatient basis under light general anesthesia approximately 6 months after repair (101 patients); the mean operating time was 19 minutes (± 6). There were no complications following bar removal.
RESULTS
Each of the 116 patients was contacted by telephone, e-mail, or questionnaire from 4 months to 14 years after operation (mean follow-up 4.3 years). Responses were obtained from 113 patients (97%). Three patients could not be located. One hundred nine of the 113 patients who responded to the questionnaire indicated that they considered the result following repair to be very good or excellent (96.5%), and that they would highly recommend repair of pectus deformities to other patients (Figs. 5–9). Two patients who underwent repair of recurrent PE deformities indicated only slight improvement (Fig. 10). Moderate to marked improvement in exercise tolerance with much less dyspnea and increased stamina and endurance was reported by all but three patients within 6 months after operation. Most patients were able to participate in exercises, such as running, hiking, swimming, golfing, and cycling, before removal of the sternal bar. All but 2 of the 84 patients with activity-induced discomfort or pain in the anterior chest noted considerable improvement within 4 months. The tachycardia and/or palpitations reported by 74 patients preoperatively were reduced considerably for each within 6 months after operation. Seventeen of the 19 patients with frequent respiratory infections had a decrease in the frequency and severity as well as a reduction in cough within 6 months. All but 1 of the 31 patients with exercise-induced wheezing and 12 of the 13 patients with asthmatic symptoms experienced fewer episodes of wheezing and a decrease in requirement for bronchodilator medications. All but 1 of the 11 patients with mild dysphagia noted improvement within 4 months.
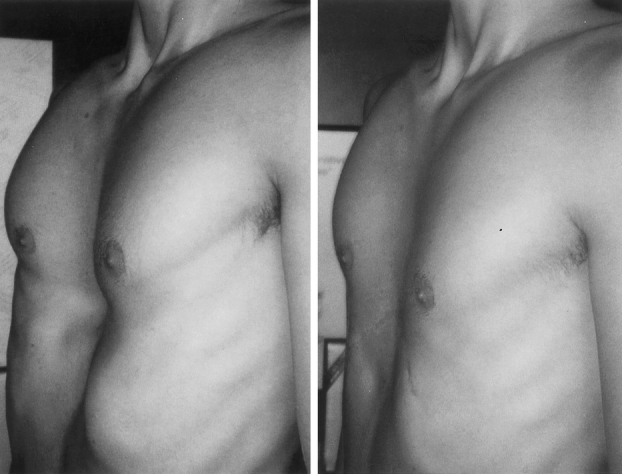
Figure 5. A 19-year-old male with pectus severity index of 4.22. Postoperative appearance 1 month following removal of sternal bar.
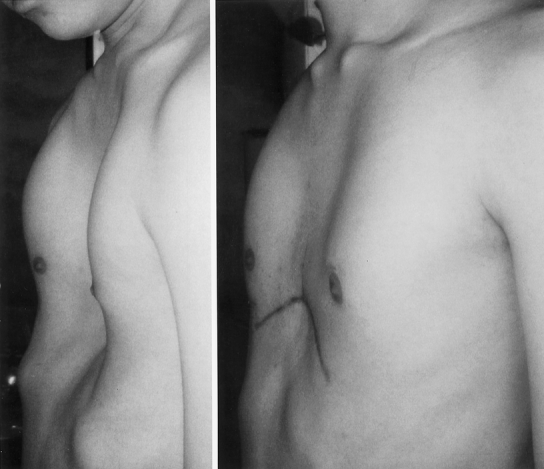
Figure 6. A 23-year-old male with pectus severity index of 9.2. Chest appearance 5 months after operation.
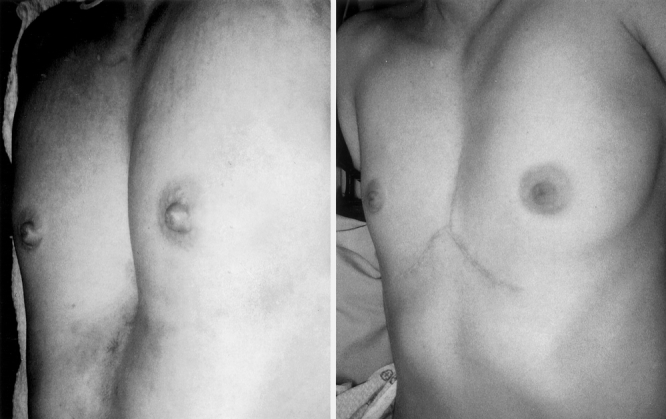
Figure 7. A 35-year-old female with pectus severity index of 7.51. Chest appearance 2 months after removal of sternal bar.
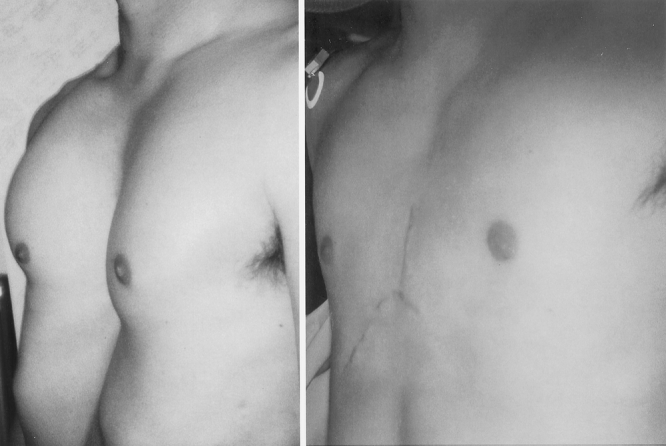
Figure 8. A 32-year-old male with pectus severity index of 7.4. Appearance 4 months following removal of sternal bar.
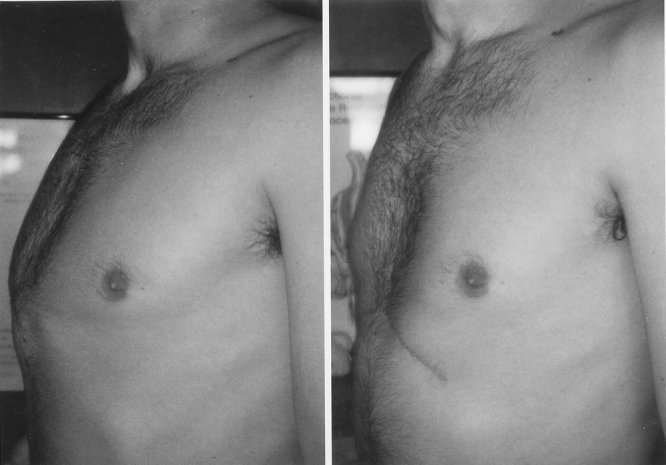
Figure 9. A 26-year-old male with pectus carinatum (severity index 1.46). Chest appearance 3 months following removal of sternal bar.
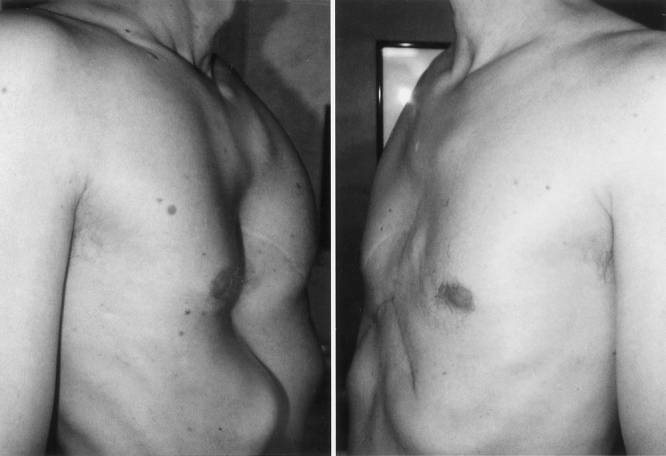
Figure 10. A 52-year-old male with recurrent pectus excavatum, 33 years following initial repair (severity index 3.81). Chest appearance 5 months following removal of sternal bar.
Although objective measurements of physiologic improvement following operation are not available for all patients, all but one of the PE patients showed a shifting of the heart from the left chest to a normal position on chest radiographs within the first week after operation. Eighteen of the 19 patients who underwent preoperative measurement of vital capacity with an incentive spirometer experienced improvement within 6 months (mean improvement 10.6%). Within 6 months functional heart murmurs were no longer audible in 16 of 26 patients.
Postoperative complications included pleural effusion in seven patients, atelectasis or pneumonitis in four patients, and unintentional left pneumothorax in two patients. In two patients with severe sternal depression (severity index > 7.0), pericarditis with pericardial effusion developed in the early postoperative period. Complete resolution occurred in both within 3 weeks after treatment with indomethacin and other nonsteroidal antiinflammatory medications. Mild irregularity of the anterior chest was observed in six patients within 1 year after the sternal bar was removed; three of them underwent minor revision on an outpatient basis. A mild recurrent depression occurred in two patients, neither of which produced symptoms sufficient to warrant any reconstruction. The sternal support bar became dislodged in three patients within 4 months, causing mild pain in each and a 20% pneumothorax in one patient. The sternal bar was removed from each of these patients from 3 to 4.5 months after operation. Mild to moderate hypertrophy of the cutaneous scar occurred in the wounds of 17 patients. It is our standard practice to inject triamcinolone solution (10 mg/mL) into any hypertrophic scar when identified and into the majority of scars at the time of sternal bar removal. There were no perioperative deaths during the period of follow-up (mean 4.3 years).
DISCUSSION
Symptoms from pectus chest deformities are uncommon during early childhood unless the patient is one of the few in whom the depression or protrusion is unusually severe. During the adolescent years of rapid skeletal growth, both PE and PC deformities almost always become much more severe at a time that coincides with increasing athletic activity. Pectus patients commonly try harder to keep up with their peers, using wider diaphragmatic excursions to compensate for diminished chest wall excursions caused by the PE or PC deformity; however, almost all patients experience a worsening of their symptoms. The deformity continues to worsen until skeletal growth has been completed in late adolescence and then changes little throughout adult life. Dyspnea with mild exercise and decreased endurance are experienced by almost all patients with severe deformities. Many have a compression-like discomfort in the anterior chest, exercise-induced wheezing, tachycardia, and palpitations. It is clear that the deformities are not merely a cosmetic problem or that surgical repair is dangerous, minimally effective, and unnecessary, as has been advised by many well-meaning but uninformed pediatricians, family physicians, and surgeons. The availability of several web sites on the Internet regarding pectus deformities during the past few years has allowed patients to become better informed and to obtain advice from other patients and knowledgeable physicians.
No consensus has been achieved over the past few decades regarding the degree of cardiopulmonary impairment that occurs with pectus deformities and how much improvement results after surgical repair. 12 Deviation of the heart into the left chest, with compression of the right ventricle by the depressed sternum, can be very dramatic. The distance between the sternum and spine was less than 5 cm in 26% of PE patients; in four patients the distance was less than 3 cm. This distance is approximately 10 cm in most normal adults. The significance of cardiac compression and displacement on older patients with progressive coronary arterial stenosis and other cardiovascular disorders is largely speculative. Objective measurements to document the severity of physical limitations caused by PE and PC have been imprecise and often confusing. Therefore, the decision to operate was based largely on the severity of the pectus deformity as noted on physical examination and the pectus severity index, as well as the severity of symptoms, rather than on pulmonary function studies.
Ravitch 13 in 1951 noted that in occasional adults with uncorrected PE, the symptoms may progress to severe incapacity with exercise intolerance and cardiac failure, all of which may be dramatically improved by surgical repair. He cited several reports of patients who died with dyspnea and cardiac failure and in whom at autopsy no abnormality could be found except for a severe PE. 14 Patients in the present study confirm these early observations that adults may experience marked symptomatic improvement following pectus repair.
Many improvements in the technique for surgical correction of PE and PC have evolved during the eight decades since the first repairs were performed. A variety of modifications of the Ravitch technique for pectus repair, originally described in 1949 15 and later revised in 1958, 7 were used widely by most surgeons until 1997, when the MIRPE was described by Nuss et al. 4 The MIRPE avoids cartilage resection and sternal osteotomy by placing a convex steel bar under the sternum through bilateral thoracic incisions and then forcibly turning the bar over to elevate the deformed sternum and costal cartilages to the desired position. The bar is left in place for 2 to 4 years, depending on the age of the patient and the severity of the deformity. Reports of favorable clinical experience with the MIRPE have included primarily young children. 16 During the ensuing years, the MIRPE has been extended to older patients, including occasional adults. Although there was initial great enthusiasm by both patients and surgeons for the MIRPE, some recent reports have indicated that although the operating time is shorter than with the HMRR, the complication rate, length of hospitalization, and severity of postoperative pain may be considerably higher with the MIRPE. 17
Recent studies have shown that the force necessary to elevate the sternum of PE patients to the desired level correlates directly with the age of the patient and is higher for those with a severity score over 5.0. 18 Whereas children under 11 years required a mean of 15.3 pounds, adults 19 years and older required a mean of 41.2 pounds. The sternal osteotomy and resection of short segments of deformed costal cartilages medially and laterally during the HMRR reduce this force to less than 1.5 pounds, regardless of age or pectus severity. The HMRR as performed in the present study therefore appears particularly suited for adolescent and adult PE patients, who would otherwise require very high forces for sternal elevation.
Although the technical aspects of pectus repair are more tedious in adults than in children, the postoperative recovery and the long-term results have been similar. The costal cartilages are usually thicker and more brittle, and occasionally they must be scooped out from the perichondrial sheaths with a rongeur rather than being stripped out with a small elevator. As with children, a single Adkins substernal support strut has provided immediate stability to the chest that has eliminated paradoxical respiratory movements and helped to minimize chest pain and atelectasis.
Martinez et al. 19 have cautioned that removing large segments of costal cartilage in young children may interfere with the rib growth plates and produce a narrow chest. Haller et al. have also cautioned against extensive cartilage resection in children because of the occasional occurrence of constricting asphyxiating thoracic dystrophy. 20 By removing only short segments of the costal cartilages adjacent to the sternum and also laterally at the position where the chest wall is in the most anterior position, a major portion of the costal cartilages can be preserved and can be easily elevated to the desired position while increasing chest wall stability. For badly deformed cartilages, another small segment may be removed in the midportion to permit a nearly flat reconstruction. Minimizing injury to the perichondrial sheaths during removal of cartilage segments is considered essential in order to permit maximum cartilage regeneration. Placing finely minced fragments of fresh autologous cartilage into the empty perichondrial sheaths appears to enhance costal cartilage regeneration and has not increased the risk of infection. Additionally, detachment of more than the two lowermost perichondrial sheaths from the side of the sternum is not necessary in order to gain adequate access to the retrosternal space to perform the sternal osteotomy and place the support bar. Detachment of more perichondrial sheaths increases the risk of irregular cartilage regeneration and incomplete attachment between the ribs and the sternum. Resection of the xiphoid is avoided since it provides a consistent and effective method of reattaching the abdominal muscles to the lower sternum. Although the period of follow-up in the present group of patients is short, we observed no abnormalities in cartilage regeneration to form a stable and strong new rib within 6 weeks, and we attribute these results to the preservation of all perichondrium and the use of autologous cartilage chips.
Placement of the sternal support bar obliquely across the lower anterior chest appears to provide optimal support for the sternum at the desired level and may reduce the likelihood of late depression of the upper anterior chest. We have noted that placing the bar posterior to the costal cartilages or perichondrial sheaths before attaching to the appropriate rib on each side elevates the anterolateral chest as well as the sternum to provide optimal cosmetic as well as physiologic reconstruction. The support bar is used for all patients since it prevents paradoxical respiration, reduces pain, permits early ambulation, permits deeper respirations, reduces hospitalization and cost, and provides very good long-term results. For PC patients, securing the bar anterior to the sternum with a large absorbable suture provides stability. We have not found it necessary to use more than one sternal support bar in any patient.
The HMRR technique used on almost all patients in the present study has been very versatile and applicable to patients of all ages and with all types of deformities, including those with recurrent defects. 11 Minimal costal cartilage resection provides more stability to the chest during the early postoperative period and more consistent elevation of the anterior chest lateral to the sternum than when more extensive cartilage excision is performed. Furthermore, there has been only mild postoperative pain, few complications, rapid recovery with short hospitalization, rapid return to physical activities, and consistent very good to excellent results compared to techniques used previously. 6
Data from the present clinical experience indicate that many patients who do not undergo repair of severe pectus chest deformities in childhood will experience worsening symptoms in adult life. Although few corrective operations have been performed on adults previously, it appears that those adults who have symptoms and activity limitations related to the pectus deformity can undergo repair with low morbidity, low cost, short limitation of activity, and a high frequency of symptomatic improvement. Repair of pectus deformities is technically easier and is therefore encouraged during childhood; however, for those patients who did not undergo operation as children, repair during adult years should be considered as a recommended treatment option.
DISCUSSION
Dr. J. Alex Haller, jr. (Baltimore, MD): My compliments to Dr. Fonkalsrud for a beautifully presented paper, and my thanks for allowing me to see the manuscript before the meeting. You are going to greatly enjoy reading this manuscript because it makes some very important points about the management of this congenital abnormality in the adult.
One of the most important features is that the indications for operative repair in this group of patients are symptoms of dyspnea and cardiovascular dysfunction rather than cosmesis. I think this should be one of the most practical aspects of the paper, that for the first time there is clear indication that the primary indication is the symptomatic patient.
As you have heard, I have been given permission to show three slides of the same patient, because I want to base my comments upon these, although you have seen some good examples from Dr. Fonkalsrud’s paper.
This is a 36-year-old man who also has Marfan’s syndrome. He has a very large ascending aorta and will be a candidate for aortic root replacement. You can see how deep his pectus is, and notice that the concavity is shifting toward the right side, which is also characteristic. The next slide shows another view of the same patient and shows how narrow the chest is in addition to the depth of the concavity. The third slide is the CT scan on this patient, which I particularly wanted to share with you because it shows that the sternum is practically on the vertebral processes. This shifts the heart into the left chest.
And I am sorry that Dr. Ravitch did not live a few more years to have heard the papers recently from Poland which show that for the first time there is no question but that displacement of the heart and the posterior position of the sternum impedes the outflow of the right ventricle. These echocardiographic studies show that this is the cardiovascular defect associated with pectus excavatum. That is why these patients, I think, were symptomatic. As Dr. Ravitch suspected, there is a functional problem, but he did not have the technology prove it.
I would like to make two more comments related to this series.
The surgical procedure is beautifully illustrated, but I think the point made about the symptomology is more important for all of us and should make us focus then upon when we should operate on children who have pectus excavatum. Since severity of the pectus excavatum increases with time, and since the only recurrences from operations that are done in children take place during the pubertal growth spurt, Dr. Fonkalsrud, should we be waiting to operate on patients, children with pectus excavatum, until they are in early stages of their pubertal growth, therefore decreasing the chances of recurrence but giving them the same good long-term results?
Do you have other evidences of compression of the right side of the heart in any of your studies? You did not include those in your manuscript, but I hope you might share those observations with us.
Presenter Dr. Eric W. Fonkalsrud (Santa Monica, CA): Dr. Haller has been one of my mentors since I was a resident, and I always learn from him. He has contributed greatly to our understanding of the surgical management of patients with chest wall deformities. Not only did his photograph of the patient show the pectus deformity but also the characteristic narrow anterior to posterior diameter of the chest, which limits the expansion of the chest further and requires that the patient use the diaphragms more actively during respiration movements. The CT scan that you showed with a 3- to 6-cm distance between the sternum and spine is similar to that in many patients. We have four adult patients where the distance was less than 3 cm. The normal chest has a distance of about 10 cm between the sternum and spine.
Regarding compression by the sternum and the effects on the heart, it is common for patients to experience compression of the right ventricular outflow tract. Over 20% of our patients had a murmur, which in many disappeared or became less prominent following repair. There is a high frequency of associated mitral valve prolapse, which may possibly be related to the compression and disfigurement of the heart by the depressed sternum. Although this is speculative, there are a few patients who had no further evidence of mitral valve prolapse after repair. The effect of right ventricular compression on an adult who later develops coronary vascular disease or other pulmonary disorders is only speculative.
Dr. Marshall Z. Schwartz (Wilmington, DE): As the surgeon that performs most of the anterior chest wall corrections at my institution, I also know that they can be long and tedious procedures. This is especially true in the older patients. Therefore, I wish to congratulate Dr. Fonkalsrud on his superb results in a large number of patients. I have a few questions that I would like to raise.
How and why do you use the pectus severity score? I have not found this to be particularly helpful and certainly not different than what you can see visually. I am not sure that there is any prognostic value to this score.
Your average hospital stay was remarkably short, 2.9 days on average. This is about a day shorter than I am able to get most of the patients out of the hospital. What do you use for pain medication on discharge? And if you leave drains in these patients, do you always take them out before they are discharged?
Finally, I notice that you remove the pectus bar in almost all of the patients at around 6 months. I have not done this. I have found that the only patients in which I remove the bar is if it migrates. Bar migration occurs in less than half of the patients over a many-year follow-up. Thus, I am curious to know why you removed them in most of your patients.
Dr. Eric W. Fonkalsrud (Santa Monica, CA): We use the severity index primarily to compare one patient with another and let the patient know how severe their defect actually is. Patients often ask, “Is this severe enough to do something about?” If we indicate that the average score for patients on whom we have operated is between 4.4 and 4.8, and the new patient has an index of 4.5 or so, he/she is usually convinced that there is indeed a severe deformity. But, as Dr. Haller pointed out, our main indication for operation is the severity of symptoms and not the cosmetic appearance. With respect to pain, I suspect that the sternal bar does much to minimize the paradoxical respiratory movements of the chest following repair. Most can get by with low intravenous doses of Dilaudid or Demerol, often regulated by a PCA. After 48 hours almost all patients receive adequate analgesia with oral Vicodin. We have found that a clinical pathway is very helpful in managing pectus patients. Adult patients go home with 40 tabs of Vicodin, with rare requests for refill. Postoperative pain is remarkably mild.
With respect to drains, we routinely use a right chest tube, placed at the time of repair. The majority of fluid is removed during the first 24 hours. The rest will be absorbed from the right chest within the next few weeks. A drain in the submuscular space often does not adequately remove the fluid accumulation, which may take weeks to resolve. Repeated aspirations may then be needed, which may increase the risk of infection. We remove the sternal bars in 6 months because when cartilage chips are placed around the end of the bar and into the perichondrial sheaths, it becomes difficult to remove the bars if one waits longer. The chest is very stable in 6 months. Cartilage regenerates within 6 weeks to 2 months in the majority of patients, and most have a stable chest at that time.
Regarding the optimal age for operating on patients with pectus excavatum, I agree with Dr. Haller that we should be cautious in operating on prepubescent children, who often have minimal symptoms. Early operation can alter the growth plates in the tips of the cartilages. Only the most severe deformities in symptomatic patients should be repaired in early childhood, perhaps using the new minimally invasive technique.
Dr. James B. D. Mark (Stanford, CA): I enjoy listening to Dr. Fonkalsrud speak because I always learn something from him. We did a fair number of pectus repairs in children until our pediatric surgeons came along, and with some regret we gave up operating on children with pectus excavatum and carinatum. But now that I hear that Dr. Fonkalsrud is operating on adults, maybe I ought to rethink that position. There are a couple of questions I have, Dr. Fonkalsrud.
It is nice to think that there is really a functional problem associated with pectus excavatum. You have presented symptoms that your patients had. But is there any other documentation of limitation of pulmonary or cardiac reserve that would allow us to better say to this patient, “You are going to be better physiologically after this operation”? I think that is particularly important, as you well know, for children. Parents are very protective of their children when it comes to doing major operations.
I also notice that you have far more males than females, at least in your pictorial review. My experience is that boys like pectus excavatum repaired more than girls. Boys hesitate to go around with their shirts off. Girls are usually more covered than boys—at least they were when I was a lad. I wonder if this has been your experience as well.
Dr. Eric W. Fonkalsrud (Santa Monica, CA): The frequency of pectus deformities is about five to six times higher in males than females. Until the last few years, females have been somewhat reluctant to seek repair. The Internet discussions may have been helpful in providing some concept of how limited patients of both sexes have been in their activities. As women are becoming more active in sporting and other activities, they are finding increasing limitations caused by the deformity. The breasts tend to fill the depression in the excavatum patients, and consequently many patients and physicians are not aware that several women may have severe deformities. Chest x-ray or CT scans will clarify the severity of the deformity. With respect to the physiologic data, it has been very difficult to obtain pulmonary function studies under exercise conditions in a prospective manner. Insurance companies will rarely cover the cost of both pre- and postoperative studies, which are expensive. It has been demonstrated in several different hospitals, including our own, that the total lung capacity, the maximum voluntary ventilation, the total exercise time, the maximum oxygen uptake, and the maximum breathing capacity are all improved, which we indicate to the patients.
Dr. Larry R. Kaiser (Philadelphia, PA): Dr. Fonkalsrud and I actually have had an opportunity in the past to discuss the repair of these lesions. I benefit from the fact that the Hospital of the University of Pennsylvania sits next to the Children’s Hospital of Philadelphia, and the pediatric surgeons there often refer these patients to us. I recall from my training in thoracic surgery in Toronto that Alex Patterson did these cases because he seemingly lost the draw and had to do them. But I have always found them fascinating and have enjoyed doing them over the years. I have a few questions for Dr. Fonkalsrud.
First, you just mentioned the issue about performing pulmonary function studies. Often these symptoms, unfortunately, do not correlate with a restrictive defect in the pulmonary function studies; that is, we can’t demonstrate a decrease in vital capacity in these patients despite the fact they may complain of the symptoms. And the symptoms themselves are fairly soft; that is, dyspnea with mild exertion. We often have difficulty with insurers paying for this operation because the assumption is that it is a “cosmetic” procedure.
With the repair that you described, how do you deal with a rotational deformity? We have taken a little different approach, and I would ask you to comment on it. We have begun to use techniques that are well recognized in orthopedic surgery for bone fixation; that is, using plates and screws, either the AO or Synthes, that allow us to bend the plate for the appropriate correction, which provides excellent fixation, as the orthopedic surgeons have shown us, and, of course, these don’t have to be removed.
Dr. Eric W. Fonkalsrud (Santa Monica, CA): In order to be meaningful, pulmonary function tests really have to be performed under exercise conditions in pectus patients. The vast majority of function studies that are performed before we see patients are carried out at rest, and therefore vital capacity and the other measurements that we mentioned earlier are not likely to be reflective of how severely incapacitated the patient is. With respect to the rotational and asymmetric deformities, the new minimally invasive technique which places a precurved bar under the sternum, rotates it, and thus lifts the sternum outward is difficult to use in patients who have asymmetric deformities. Forty-eight percent of our adult patients had asymmetric deformities, with the depression being more severe on the right in most cases. With the highly modified Ravitch repair, the transverse wedge osteotomy across the anterior table of the sternum, leaving the posterior table intact, allows one to twist and elevate the sternum to the desired position with minimal pressure, rarely over 1.5 lb. The sternum is secured in the optimal position by placing the sternal bar obliquely across the chest to elevate the whole sternum, not just the lower tip; otherwise, the upper sternum may develop a depression. With respect to the plates and screws, I look forward to hearing more about your experience with this technique. Most patients have indicated a desire to have the hardware removed as soon as possible and tend not to do as extensive athletic activities if there is hardware still present.
Footnotes
Presented at the 122nd Annual Meeting of the American Surgical Association, April 24–27, 2002, The Homestead, Hot Springs, Virginia.
Correspondence: Eric W. Fonkalsrud, MD, Department of Surgery, UCLA School of Medicine, 10833 Le Conte Avenue, Los Angeles, CA 90095.
E-mail: efonkalsrud@mednet.ucla.edu
Accepted for publication April 24, 2002.
References
- 1.Molik KA, Engum SA, Rescorla FJ, et al. Pectus excavatum repair: experience with standard and minimal invasive techniques. J Pediatr Surg 2001; 36: 324–328. [DOI] [PubMed] [Google Scholar]
- 2.Fonkalsrud EW, Beanes S. Surgical management of pectus carinatum: 30 years’ experience. World J Surg 2001; 25: 898–903. [DOI] [PubMed] [Google Scholar]
- 3.Shamberger RC, Welch KJ. Cardiopulmonary function in pectus excavatum. Surg Gynecol Obstet 1988; 166: 383–391. [PubMed] [Google Scholar]
- 4.Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction or pectus excavatum. J Pediatr Surg 1998; 33: 545–552. [DOI] [PubMed] [Google Scholar]
- 5.Fonkalsrud EW, Bustorff-Silva J. Repair of pectus excavatum and carinatum in adults. Am J Surg 1999; 177: 121–124. [DOI] [PubMed] [Google Scholar]
- 6.Haller JA, Kramer SS, Lietman SA. Use of CT scans in selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg 1987; 22: 904–906. [DOI] [PubMed] [Google Scholar]
- 7.Ravitch MM. Operation for correction of pectus excavatum. Surg Gynecol Obstet 1958; 107: 619–623. [PubMed] [Google Scholar]
- 8.Welch KJ. Satisfactory surgical correction of pectus excavatum deformity in childhood: a limited opportunity. J Thorac Surg 1958; 36: 697–713. [PubMed] [Google Scholar]
- 9.Fonkalsrud EW, Dunn JCY, Atkinson JB. Repair of pectus excavatum deformities: 30 years experience with 375 patients. Ann Surg 2000; 231: 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adkins PC, Blades B. A stainless steel strut for correction of pectus excavatum. Surg Gynecol Obstet 1961; 113: 111–113. [PubMed] [Google Scholar]
- 11.DeUgarte D, Choi E, Fonkalsrud EW. Repair of recurrent pectus chest deformities. Am Surgeon (in press). [PubMed]
- 12.Shamberger RC. Congenital chest wall deformities. Curr Prob Surg 1996; 33: 469–552. [DOI] [PubMed] [Google Scholar]
- 13.Ravitch MM. Pectus excavatum and heart failure. Surgery 1951; 30: 178–182. [PubMed] [Google Scholar]
- 14.Ravitch MM. Operative treatment of congenital deformities of the chest. Am J Surg 1961; 19: 588–597. [DOI] [PubMed] [Google Scholar]
- 15.Ravitch MM. Operative technique of pectus excavatum repair. Ann Surg 1949; 129: 429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croitoru DP, Kelly RE Jr, Goretsky MJ, et al. Experience and modification update for the minimally invasive Nuss technique for pectus excavatum repair in 303 patients. J Pediatr Surg 2002; 37: 437–445. [DOI] [PubMed] [Google Scholar]
- 17.Fonkalsrud EW, Beanes S, Hebra A, et al. Comparison of minimally invasive and modified Ravitch pectus excavatum repair. J Pediatr Surg 2002; 37: 413–417. [DOI] [PubMed] [Google Scholar]
- 18.Fonkalsrud EW, Reemtsen B. Force required to elevate the sternum of pectus excavatum patients. J Am Coll Surg (in press). [DOI] [PubMed]
- 19.Martinez D, Juame J, Stein T, et al. The effect of costal cartilage resection on chest wall development. Pediatr Surg Int 1990; 5: 170–173. [Google Scholar]
- 20.Haller JA, Colombani P, Humphries C, et al. Chest wall constriction after too extensive and too early operations for pectus excavatum. Ann Thorac Surg 1996; 61: 1618–1625. [DOI] [PubMed] [Google Scholar]


