Abstract
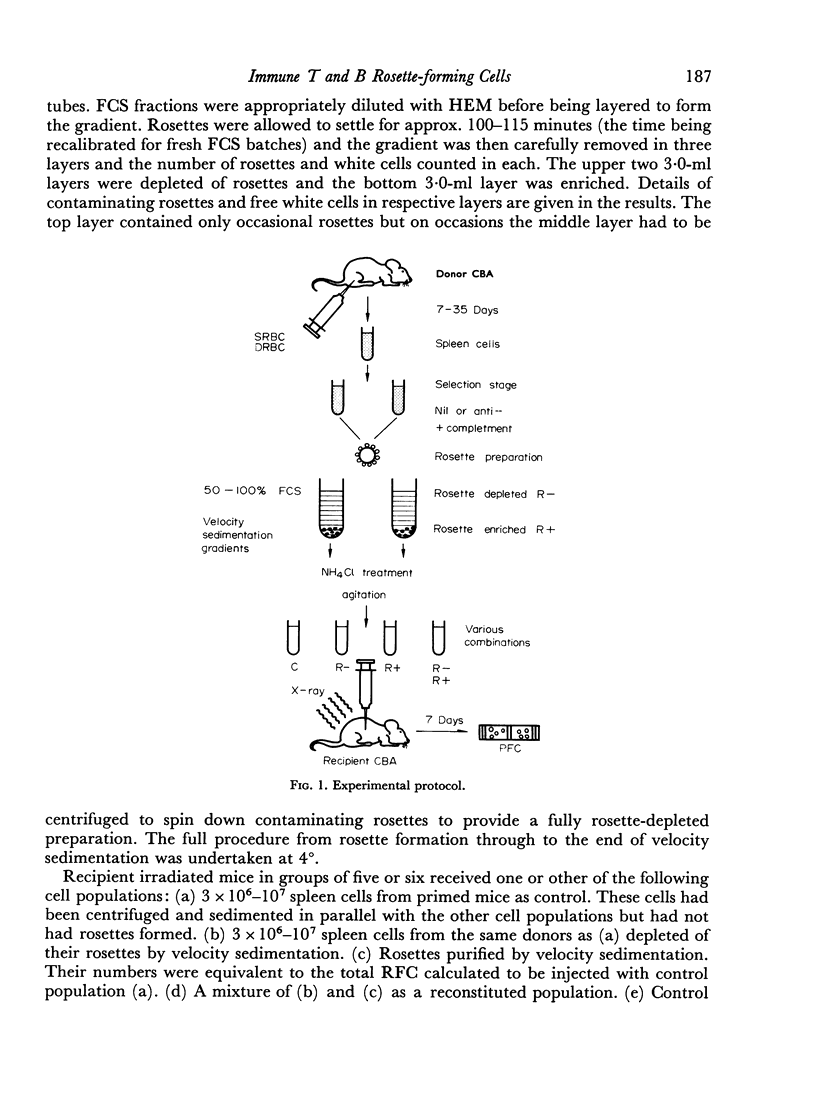
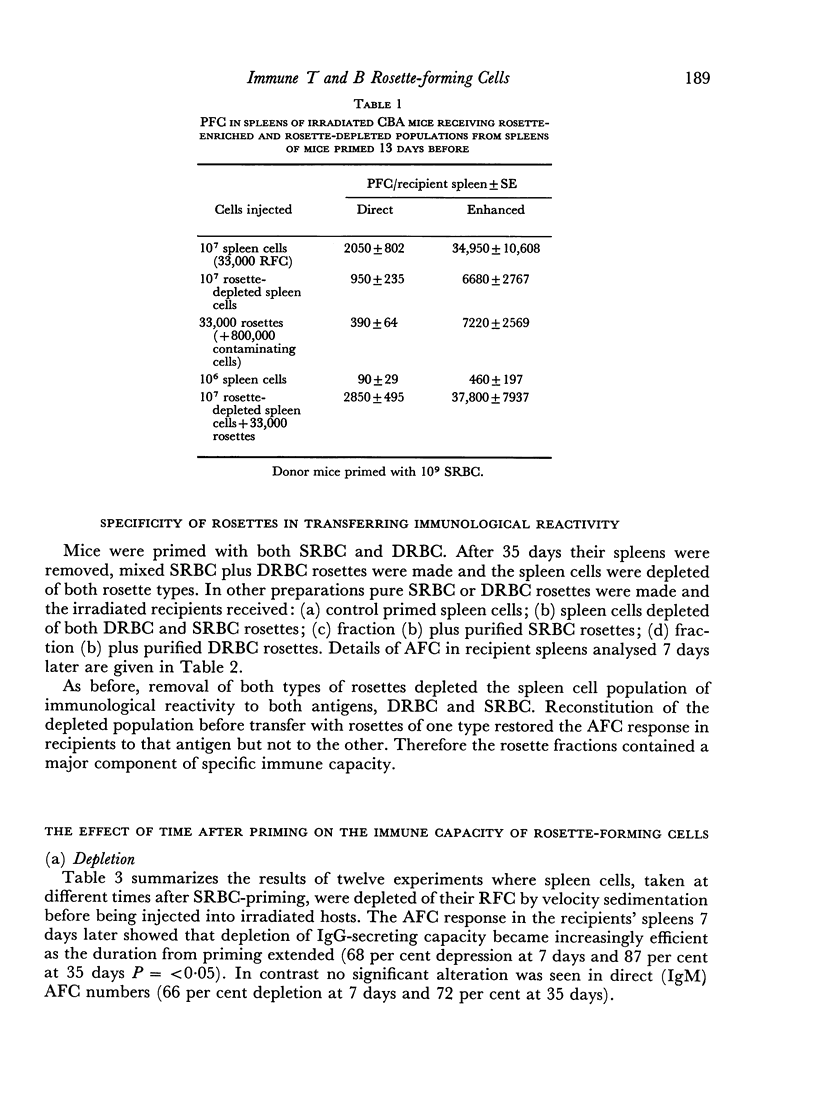
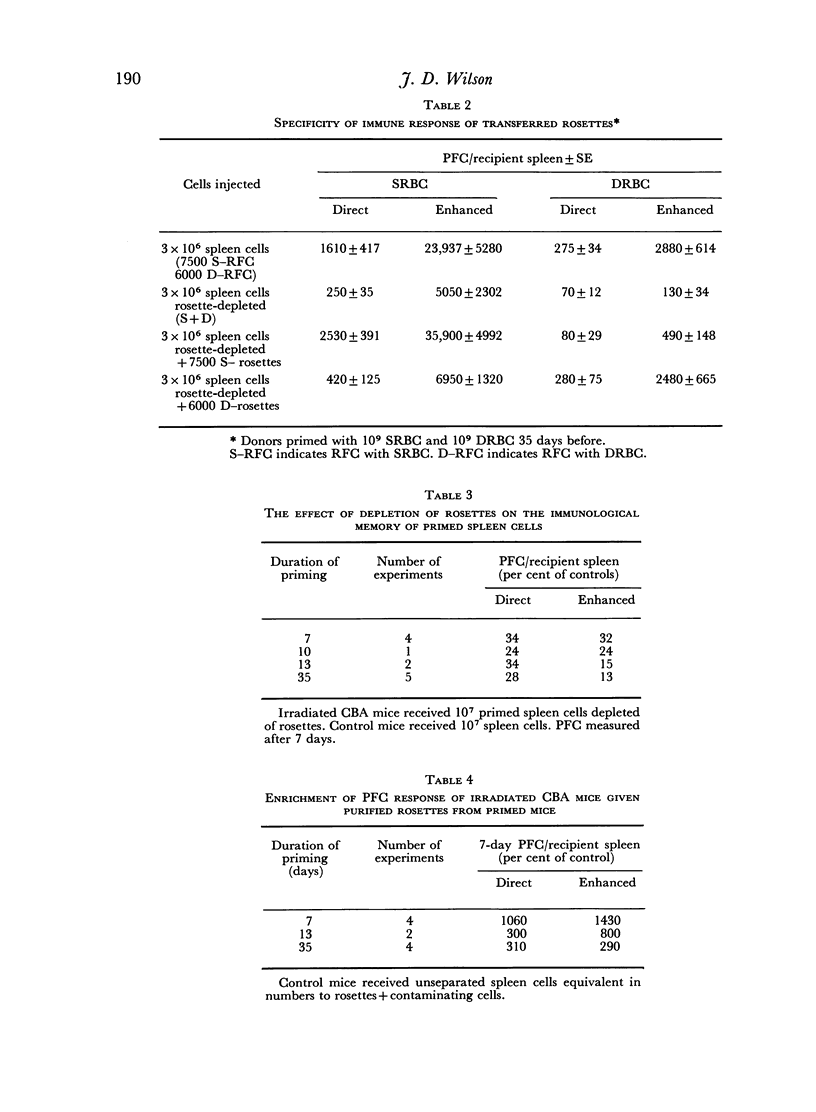
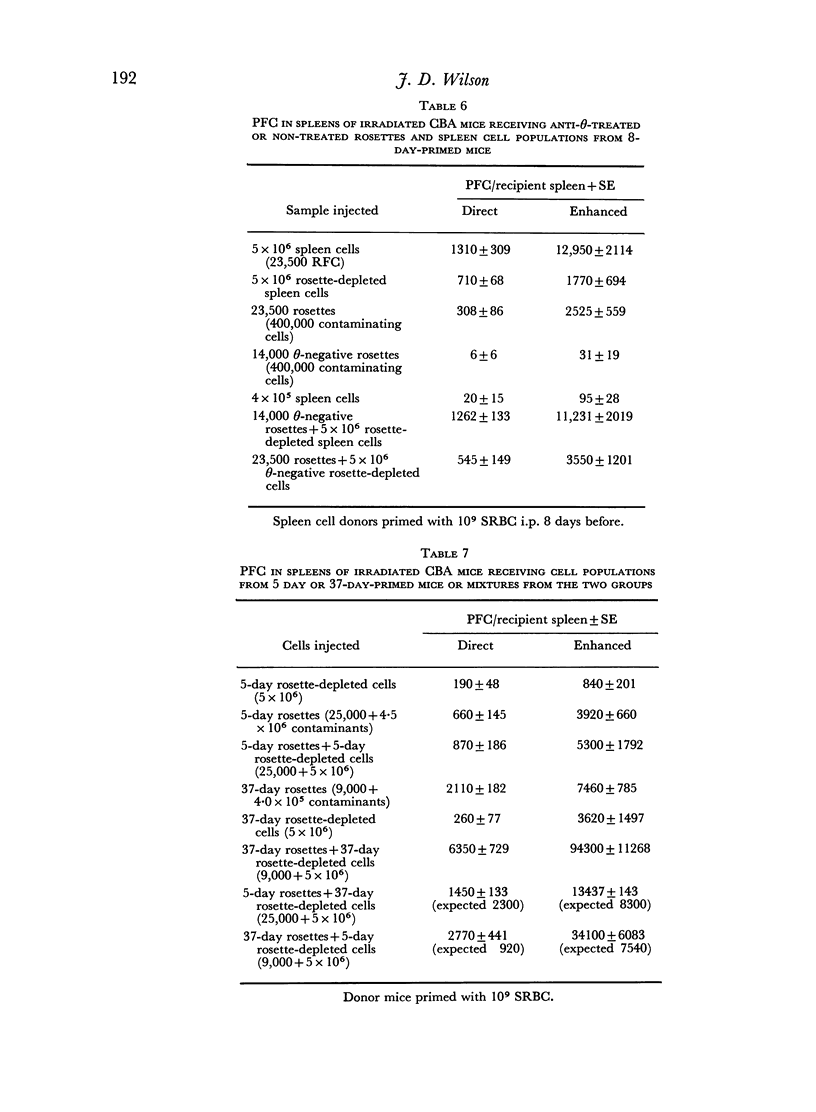
From 7 to 35 days after CBA mice were primed with SRBC their spleens were removed and anti-SRBC rosettes were formed. The rosettes were purified from other spleen cells by velocity sedimentation at 4° and rosette-enriched, rosette-depleted and various control populations were injected into lethally irradiated CBA recipients. These were challenged with SRBC and their spleens analysed for direct (IgM) and enhanced (IgG) PFC 7 days later. Removal of RFC depleted the primed spleen cells of their capacity adoptively to transfer an immune response. This depletion was antigen-specific. Purified rosettes alone prepared 7–8 days after priming transferred significant (relative to controls) immune reactivity to the irradiated recipients. Both B and T RFC were present at this stage and the response was dependent upon cell collaboration between these two populations. Later in the primary response (35 days) purified rosettes transferred negligible immune reactivity. But these RFC (90 per cent B) collaborated with rosette-depleted cells to restore full reactivity. B memory lymphocytes (AFCP) form rosettes from 7 to 35 days after immunization but T memory cells only do so for a limited stage during the peak of the primary response. The majority of T memory cells probably never form rosettes in this system. It is suggested that most T RFC may be cells mediating delayed hypersensitivity or are `passive' rosettes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach J. F., Dardenne M. Antigen recognition by T lymphocytes. Thymus and marrow dependence of spontaneous rosette forming cells in the mouse. Cell Immunol. 1972 Jan;3(1):1–10. doi: 10.1016/0008-8749(72)90220-1. [DOI] [PubMed] [Google Scholar]
- Bach J. F., Muller J. Y., Dardenne M. In vivo specific antigen recognition by rosette forming cells. Nature. 1970 Sep 19;227(5264):1251–1252. doi: 10.1038/2271251a0. [DOI] [PubMed] [Google Scholar]
- Bankhurst A. D., Wilson J. D. Detection of antigen-binding cells by combined rosette formation and autoradiography. Nat New Biol. 1971 Dec 1;234(48):154–156. doi: 10.1038/newbio234154b0. [DOI] [PubMed] [Google Scholar]
- Brody T. Identification of two cell populations required for mouse immunocompetence. J Immunol. 1970 Jul;105(1):126–138. [PubMed] [Google Scholar]
- Coombs R. R., Gurner B. W., Wilson A. B., Holm G., Lindgren B. Rosette-formation between human lymphocytes and sheep red cells not involving immunoglobulin receptors. Int Arch Allergy Appl Immunol. 1970;39(5-6):658–663. doi: 10.1159/000230390. [DOI] [PubMed] [Google Scholar]
- Crone M., Koch C., Simonsen M. The elusive T cell receptor. Transplant Rev. 1972;10:36–56. doi: 10.1111/j.1600-065x.1972.tb01538.x. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Sercarz E. E. The asynchronous development of immunological memory in helper (T) and precursor (B) cell lines. Eur J Immunol. 1971 Dec;1(6):413–421. doi: 10.1002/eji.1830010602. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Gorczynski R. M., Miller R. G., Phillips R. A. Identification by density separation of antigen-specific surface receptors on the progenitors of antibody-producing cells. Immunology. 1971 May;20(5):693–705. [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Möller E. Studies on antigen-binding cells. I. The origin of reactive cells. Cell Immunol. 1970 Oct;1(4):372–385. doi: 10.1016/0008-8749(70)90015-8. [DOI] [PubMed] [Google Scholar]
- Greaves M. F. The expression of immunoglobulin determinants on the surface of antigen-binding lymphoid cells in mice. I. An analysis of light and heavy chain restrictions on individual cells. Eur J Immunol. 1971 Jun;1(3):186–194. doi: 10.1002/eji.1830010308. [DOI] [PubMed] [Google Scholar]
- Hogg N. M., Greaves M. F. Antigen-binding thymus-derived lymphocytes. II. Nature of the immunoglobulin determinants. Immunology. 1972 Jun;22(6):967–980. [PMC free article] [PubMed] [Google Scholar]
- Hämmerling U., Rajewsky K. Evidence for surface-associated immunoglobulin on T and B lymphocytes. Eur J Immunol. 1971 Dec;1(6):447–452. doi: 10.1002/eji.1830010608. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay W. H., Mendes N. F., Bianco C., Nussenzweig V. Binding of sheep red blood cells to a large population of human lymphocytes. Nature. 1971 Apr 23;230(5295):531–532. doi: 10.1038/230531a0. [DOI] [PubMed] [Google Scholar]
- McConnell I. Antigen receptors on the surface of antibody-secreting cells. Nat New Biol. 1971 Oct 6;233(40):177–179. doi: 10.1038/newbio233177a0. [DOI] [PubMed] [Google Scholar]
- McConnell I., Munro A., Gurner B. W., Coombs R. R. Studies on actively allergized cells. I. The cyto-dynamics and morphology of rosete-forming lymph node cells in mice and inhibition of rosette-formation with antibody to mouse immunoglobulins. Int Arch Allergy Appl Immunol. 1969;35(3):209–227. [PubMed] [Google Scholar]
- Michlmayr G., Huber H. Receptor sites for complement on certain human peripheral blood lymphocytes. J Immunol. 1970 Sep;105(3):670–676. [PubMed] [Google Scholar]
- Miller J. F., Sprent J. Cell-to-cell interaction in the immune response. VI. Contribution of thymus-derived cells and antibody-forming cell precursors to immunological memory. J Exp Med. 1971 Jul 1;134(1):66–82. doi: 10.1084/jem.134.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Warner N. L., Lewis H., Sprent J. Quantitative features of a sandwich radioimmunolabeling technique for lymphocyte surface receptors. J Exp Med. 1972 Feb 1;135(2):405–428. doi: 10.1084/jem.135.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osoba D. Some physical and radiobiological properties of immunologically reactive mouse spleen cells. J Exp Med. 1970 Aug 1;132(2):368–383. doi: 10.1084/jem.132.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Sternberg M., Taylor R. B. Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature. 1970 Feb 7;225(5232):553–554. doi: 10.1038/225553a0. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. Anti-theta antibodies for detecting thymus-dependent lymphocytes in the immune response of mice to SRBC. Nature. 1970 Jun 27;226(5252):1254–1256. doi: 10.1038/2261254a0. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Bianco C., Nussenzweig V., Uhr J. W. Cell surface immunoglobulin. IV. Distribution among thymocytes, bone mrrow cells, and their derived populations. J Exp Med. 1972 Jul 1;136(1):81–93. doi: 10.1084/jem.136.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D. Immunoglobulin determinants on rosette-forming cells: their changing nature during an immune response. Aust J Exp Biol Med Sci. 1971 Aug;49(4):415–419. doi: 10.1038/icb.1971.43. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., Miller J. F. T and B rosette-forming cells. Eur J Immunol. 1971 Dec;1(6):501–503. doi: 10.1002/eji.1830010622. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. The relationship of antibody-forming cells to rosette-forming cells. Immunology. 1971 Aug;21(2):233–245. [PMC free article] [PubMed] [Google Scholar]
- ZAALBERG O. B. A SIMPLE METHOD FOR DETECTING SINGLE ANTIBODY-FORMING CELLS. Nature. 1964 Jun 20;202:1231–1231. doi: 10.1038/2021231a0. [DOI] [PubMed] [Google Scholar]


