Abstract
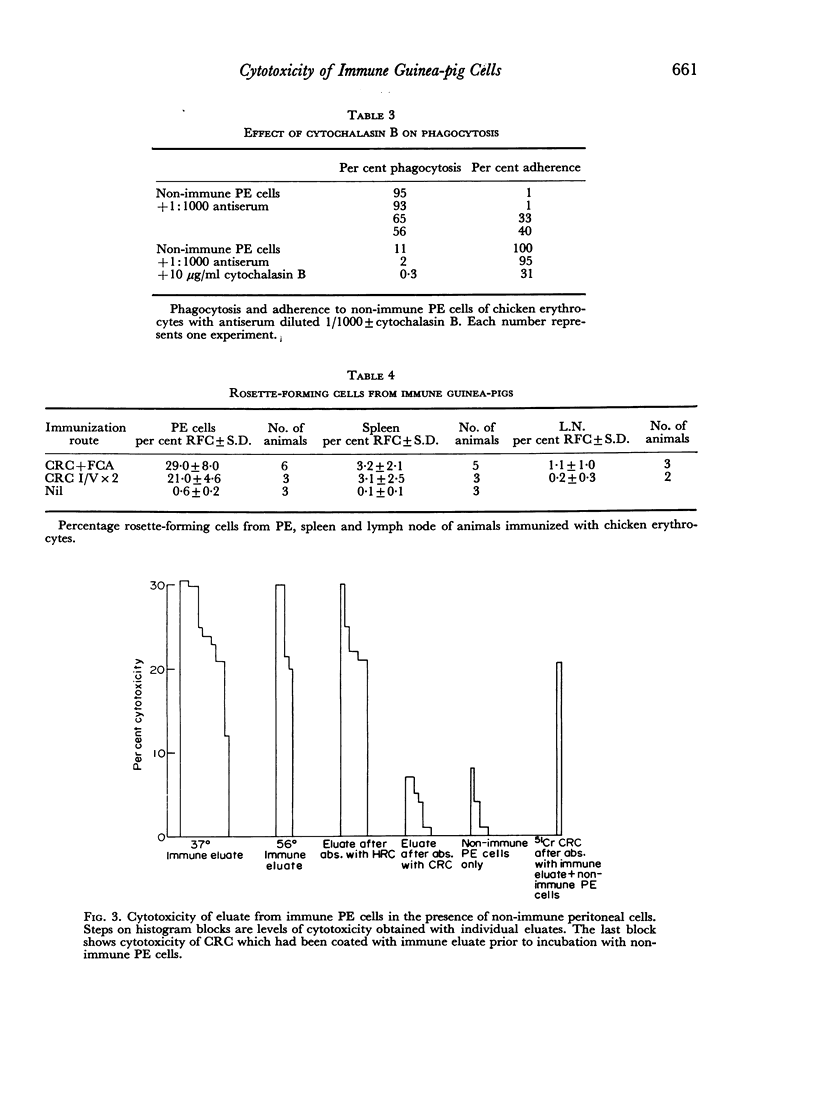
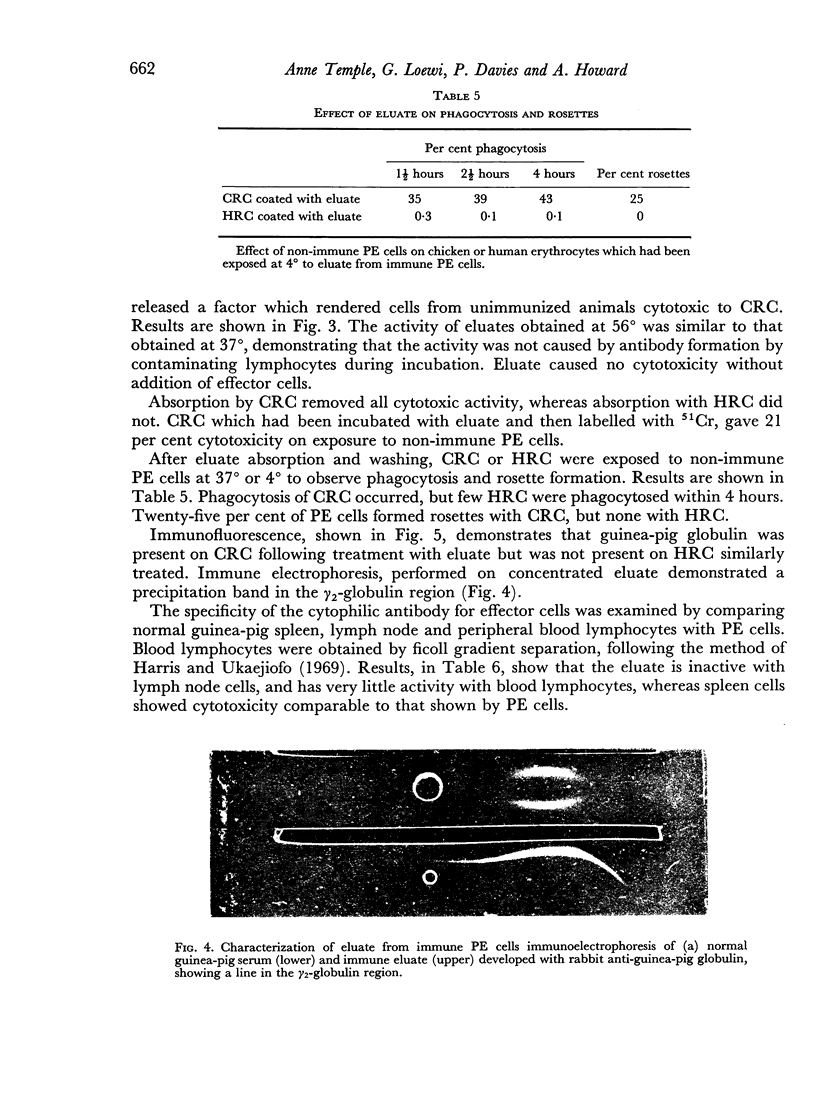
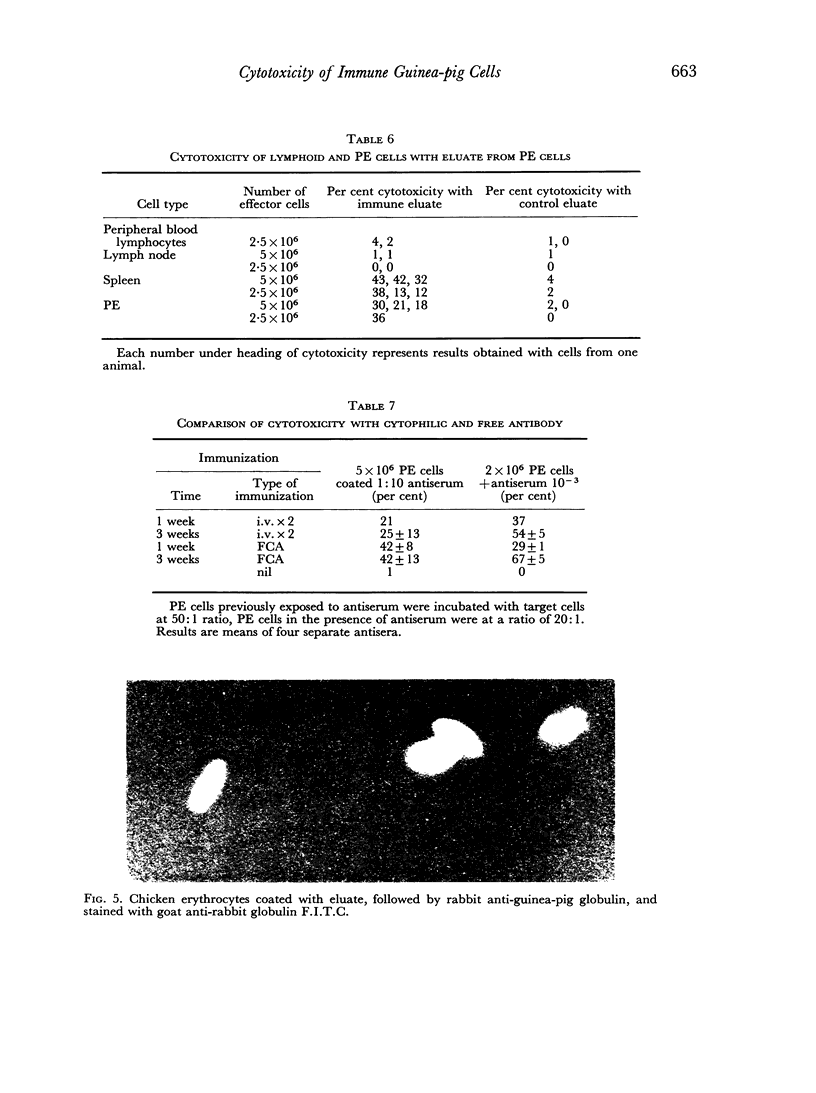
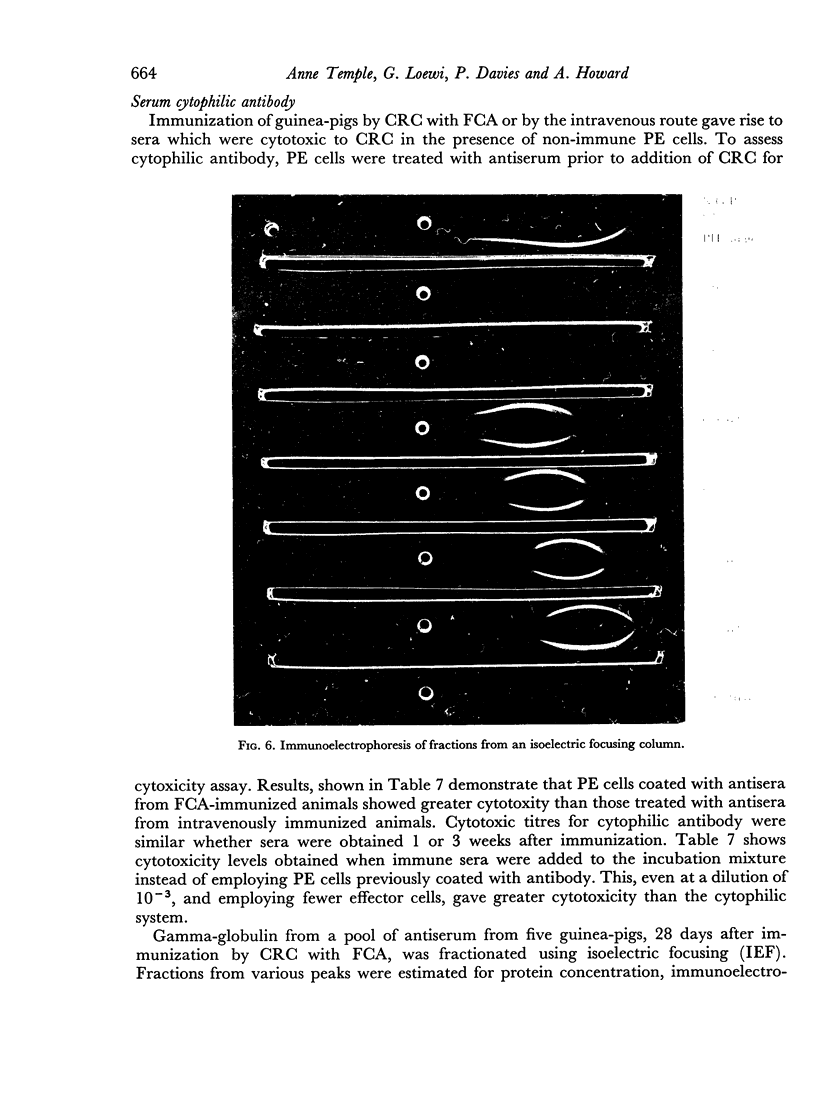
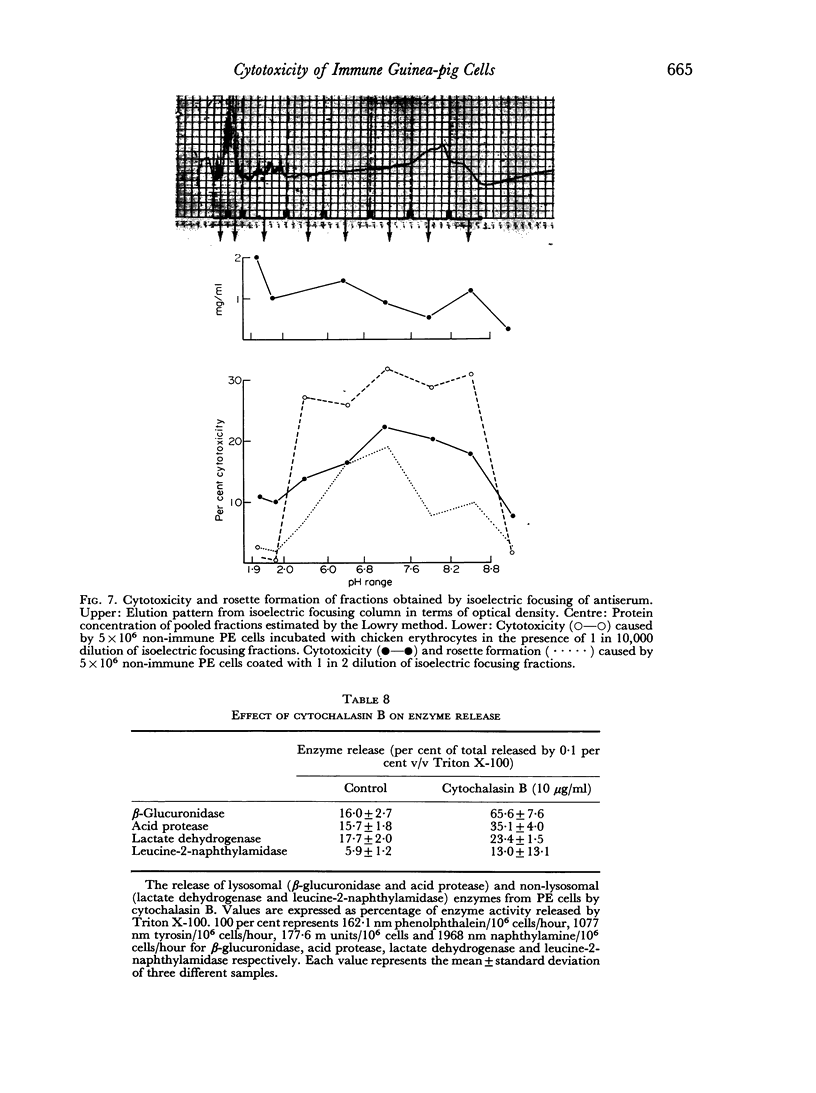
Macrophages from immune guinea-pigs, as well as macrophages from non-immune animals in the presence of specific antibody, were cytotoxic to chicken erythrocytes. Elution from macrophages produced a γ2-globulin which enabled non-immune macrophages to form rosettes with chicken erythrocytes, to phagocytose, and to show cytotoxicity towards such cells. This antibody was target-cell specific; it also rendered spleen cells cytotoxic. Isoelectric focusing of antiserum gave a peak of cytophilic antibody at pH 6.8–7.6 which proved to be in the γ2 region.
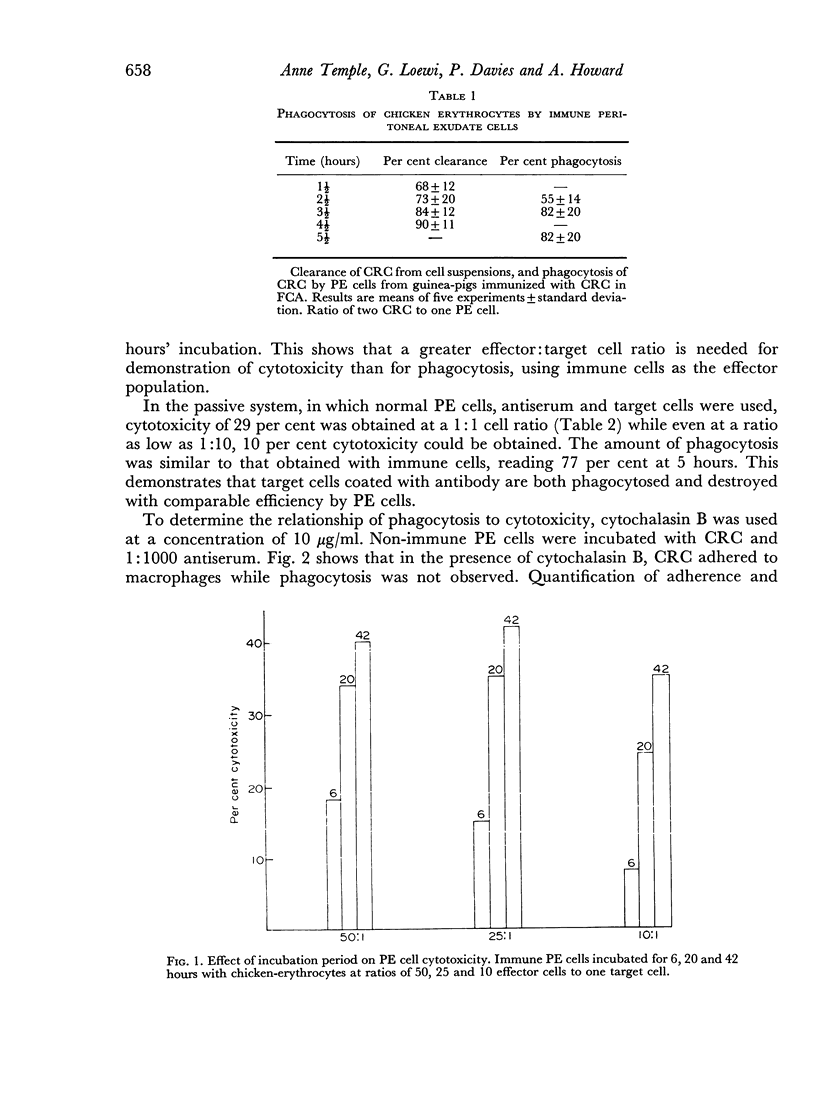
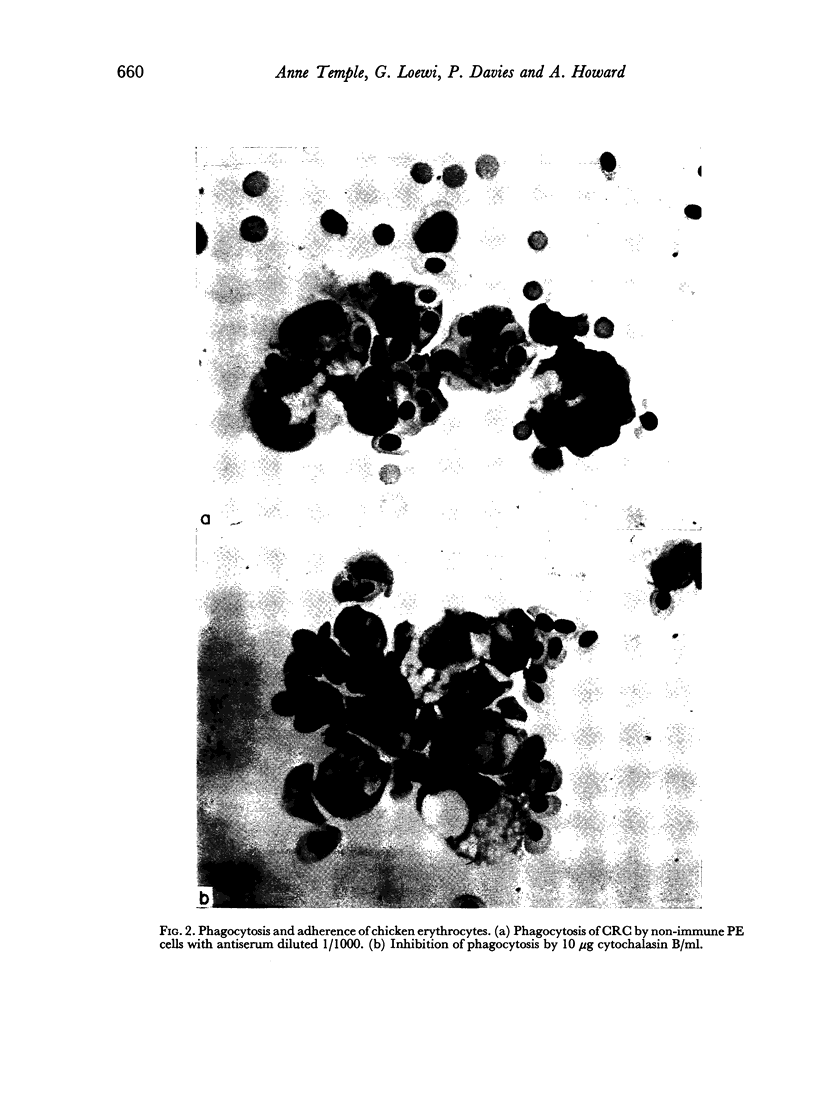
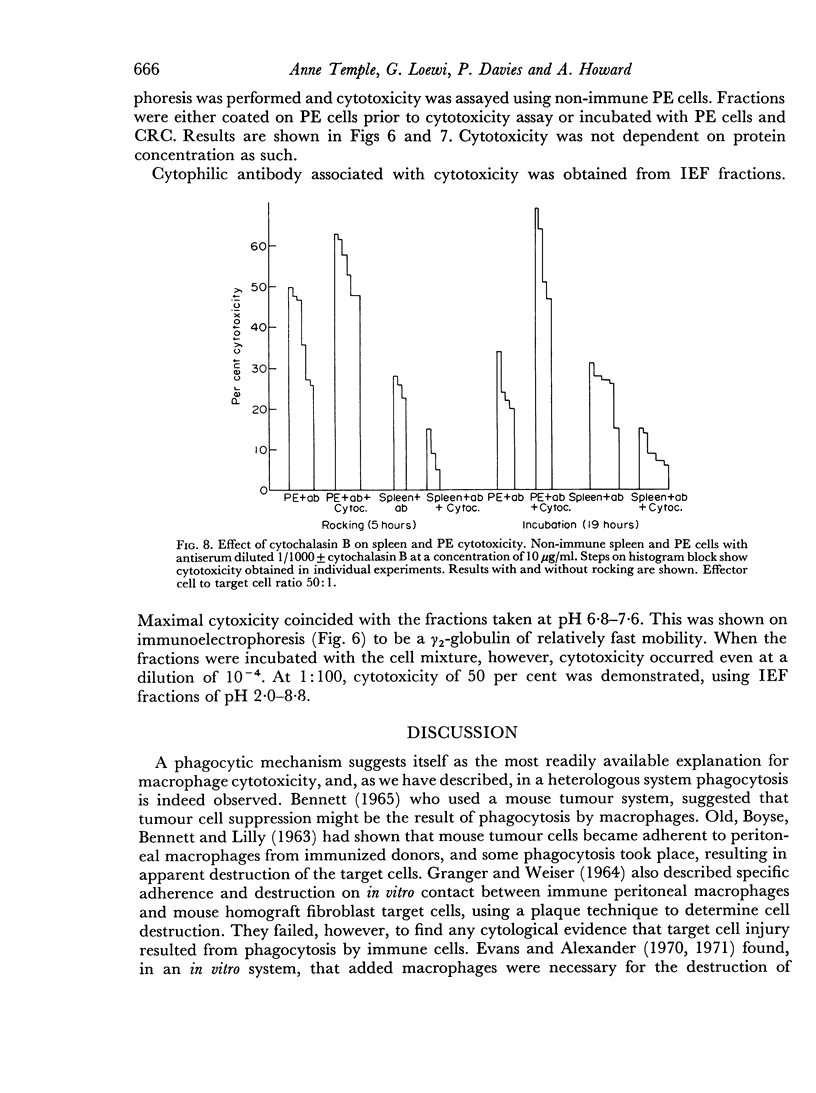
The significance of phagocytosis for the cytotoxic process was examined with the aid of cytochalasin B which inhibited macrophage—phagocytosis, but produced enhanced cytotoxicity in this sytem. At the same time there was greatly increased release of lysosomal enzymes from macrophages in the presence of target cells. It is considered that, although phagocytosis is of significance for macrophage cytotoxicity, target cell destruction can also occur by the liberation of lysosomal enzymes from the plasma membrane into the target cell when this is in close surface contact.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies P., De Petris S. Role of contractile microfilaments in macrophage movement and endocytosis. Nat New Biol. 1971 Aug 4;232(31):153–155. doi: 10.1038/newbio232153a0. [DOI] [PubMed] [Google Scholar]
- BENNETT B., OLD L. J., BOYSE E. A. THE PHAGOCYTOSIS OF TUMOR CELLS IN VITRO. Transplantation. 1964 Mar;2:183–202. doi: 10.1097/00007890-196403000-00003. [DOI] [PubMed] [Google Scholar]
- BENNETT B. PHAGOCYTOSIS OF MOUSE TUMOR CELLS IN VITRO BY VARIOUS HOMOLOGOUS AND HETEROLOGOUS CELLS. J Immunol. 1965 Jul;95:80–86. [PubMed] [Google Scholar]
- Baccino F. M., Rita G. A., Zuretti M. F. Studies on the structure-bound sedimentabolity of some rat liver lysosome hydrolases. Biochem J. 1971 Apr;122(3):363–371. doi: 10.1042/bj1220363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke G., Sullivan K. A., Amos B. Rejection of ascites tumor allografts. I. Isolation, characterization, and in vitro reactivity of peritoneal lymphoid effector cells from BALB-c mice immune to EL4 leukosis. J Exp Med. 1972 Jun 1;135(6):1334–1350. doi: 10.1084/jem.135.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berken A., Benacerraf B. Properties of antibodies cytophilic for macrophages. J Exp Med. 1966 Jan 1;123(1):119–144. doi: 10.1084/jem.123.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Allison A. C., Fox R. I., Polyzonis M., Haswell A. D. The exocytosis of polymorphonuclear-leukocyte lysosomal enzymes induced by cytochlasin B. Biochem J. 1972 Jul;128(3):78P–79P. doi: 10.1042/bj1280078p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Krakauer K., Weissmann G. Subcellular distribution of neutral protease and peptidases in rabbit polymorphonuclear leucocytes. Nature. 1970 Nov 21;228(5273):761–762. doi: 10.1038/228761a0. [DOI] [PubMed] [Google Scholar]
- Davis A. T., Estensen R., Quie P. G. Cytochalasin B. 3. Inhibition of human polymorphonuclear leukocyte phagocytosis. Proc Soc Exp Biol Med. 1971 May;137(1):161–164. doi: 10.3181/00379727-137-35535. [DOI] [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J. Uptake of biologically active substances by lysosomes. Proc R Soc Lond B Biol Sci. 1969 Apr 15;173(1030):85–93. doi: 10.1098/rspb.1969.0040. [DOI] [PubMed] [Google Scholar]
- Evans R., Alexander P. Cooperation of immune lymphoid cells with macrophages in tumour immunity. Nature. 1970 Nov 14;228(5272):620–622. doi: 10.1038/228620a0. [DOI] [PubMed] [Google Scholar]
- Evans R., Alexander P. Rendering macrophages specifically cytotoxic by a factor released from immune lymphoid cells. Transplantation. 1971 Sep;12(3):227–229. doi: 10.1097/00007890-197109000-00015. [DOI] [PubMed] [Google Scholar]
- GRANGER G. A., WEISER R. S. HOMOGRAFT TARGET CELLS: SPECIFIC DESTRUCTION IN VITRO BY CONTACT INTERACTION WITH IMMUNE MACROPHAGES. Science. 1964 Sep 25;145(3639):1427–1429. doi: 10.1126/science.145.3639.1427. [DOI] [PubMed] [Google Scholar]
- Granger G. A., Weiser R. S. Homograft target cells: contact destruction in vitro by immune macrophages. Science. 1966 Jan 7;151(3706):97–99. doi: 10.1126/science.151.3706.97. [DOI] [PubMed] [Google Scholar]
- Harris R., Ukaejiofo E. O. Rapid preparation of lymphocytes for tissue-typing. Lancet. 1969 Aug 9;2(7615):327–327. doi: 10.1016/s0140-6736(69)90096-8. [DOI] [PubMed] [Google Scholar]
- Hawkins D. Biopolymer membrane: a model system for the study of the neutrophilic leukocyte response to immune complexes. J Immunol. 1971 Aug;107(2):344–352. [PubMed] [Google Scholar]
- Hawkins D. Neutrophilic leukocytes in immunologic reactions: evidence for the selective release of lysosomal constituents. J Immunol. 1972 Feb;108(2):310–317. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Loewi G., Temple A. Cytotoxicity of immune guinea-pig cells. I. Investigation of a correlation with delayed hypersensitivity and a comparison of cytotoxicity of spleen, lymph node and peritoneal exudate cells. Immunology. 1972 Oct;23(4):559–567. [PMC free article] [PubMed] [Google Scholar]
- MacLennan I. C., Loewi G., Harding B. The role of immunoglobulins in lymphocyte-mediated cell damage, in vitro. I. Comparison of the effects of target cell specific antibody and normal serum factors on cellular damage by immune and non-immune lymphocytes. Immunology. 1970 Mar;18(3):397–404. [PMC free article] [PubMed] [Google Scholar]
- Malawista S. E., Gee J. B., Bensch K. G. Cytochalasin B reversibly inhibits phagocytosis: functional, metabolic, and ultrastructural effects in human blood leukocytes and rabbit alveolar macrophages. Yale J Biol Med. 1971 Dec;44(3):286–300. [PMC free article] [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]