Abstract
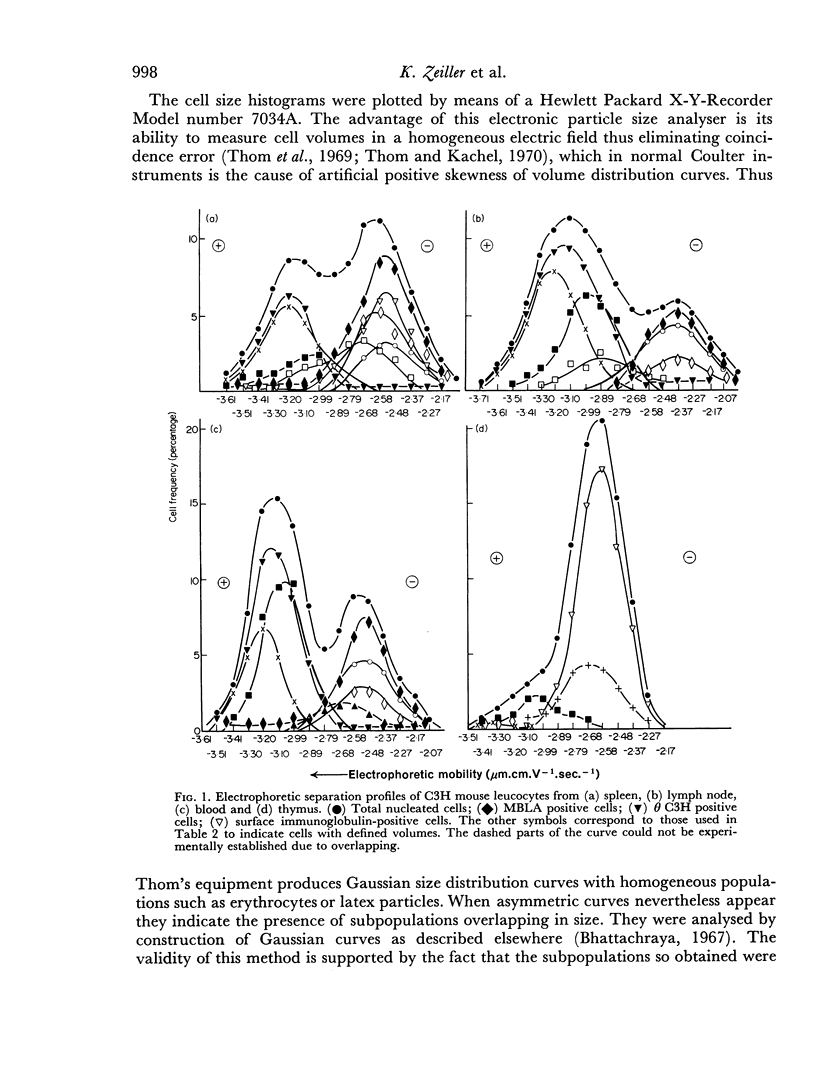
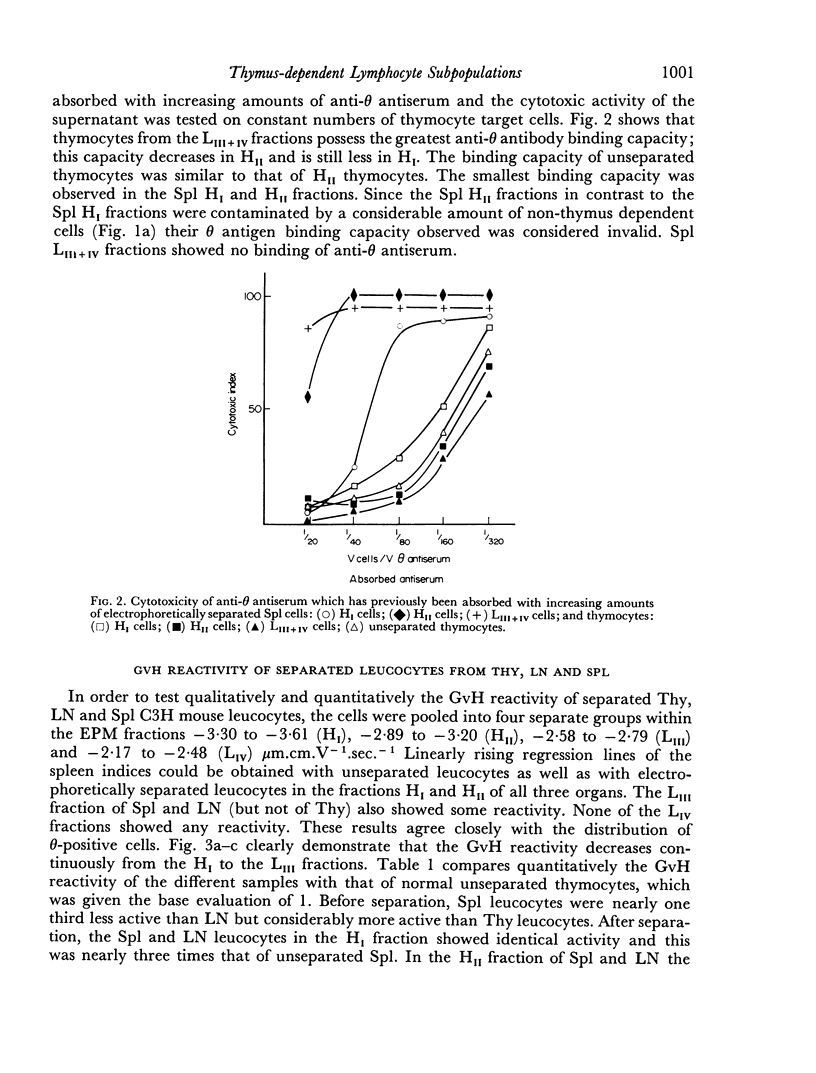
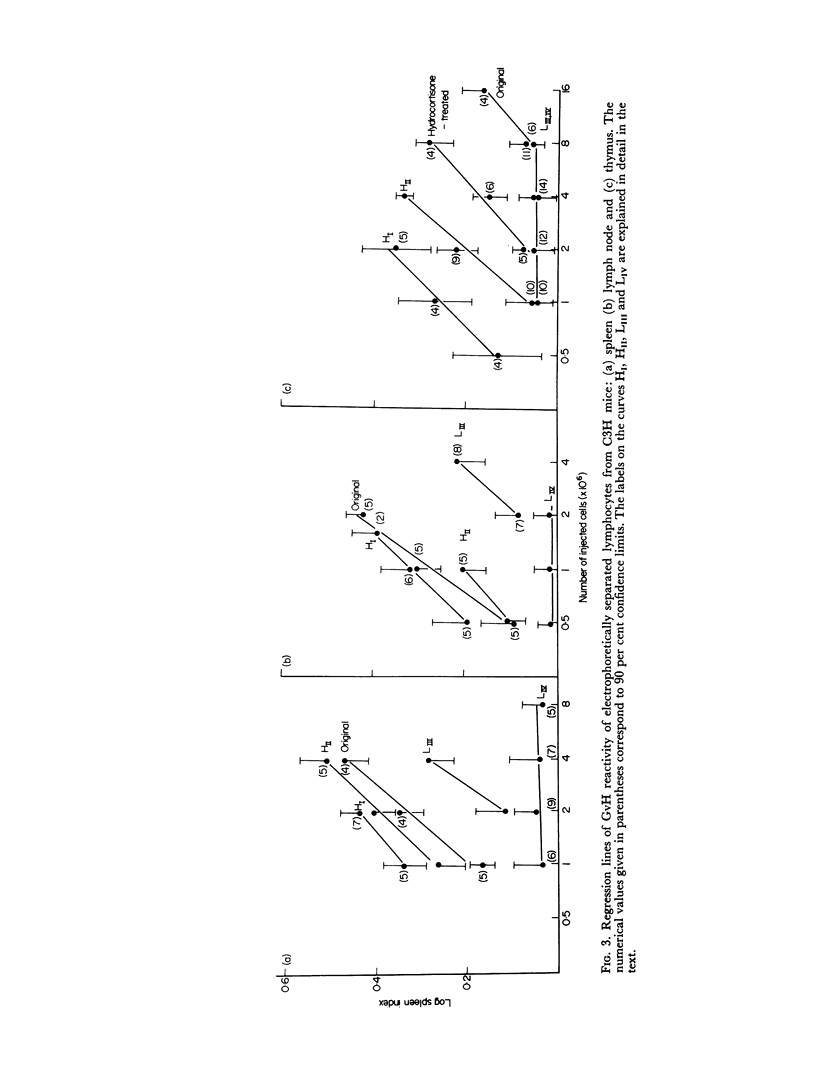
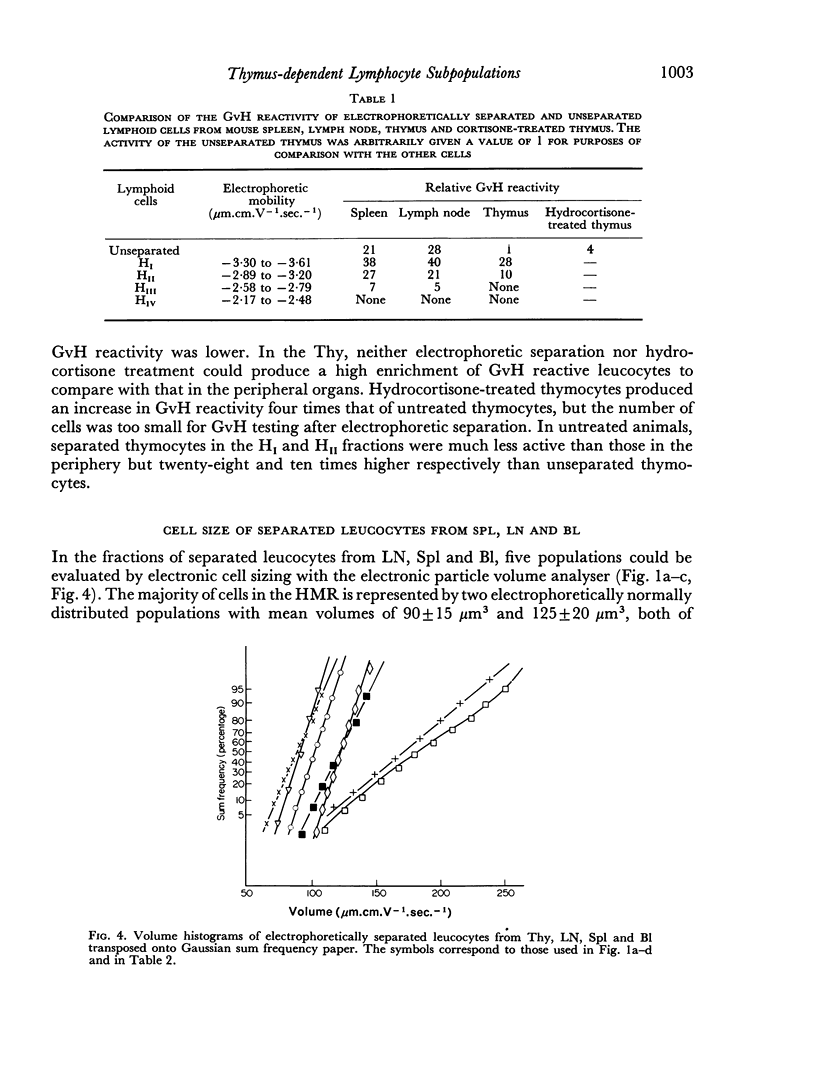
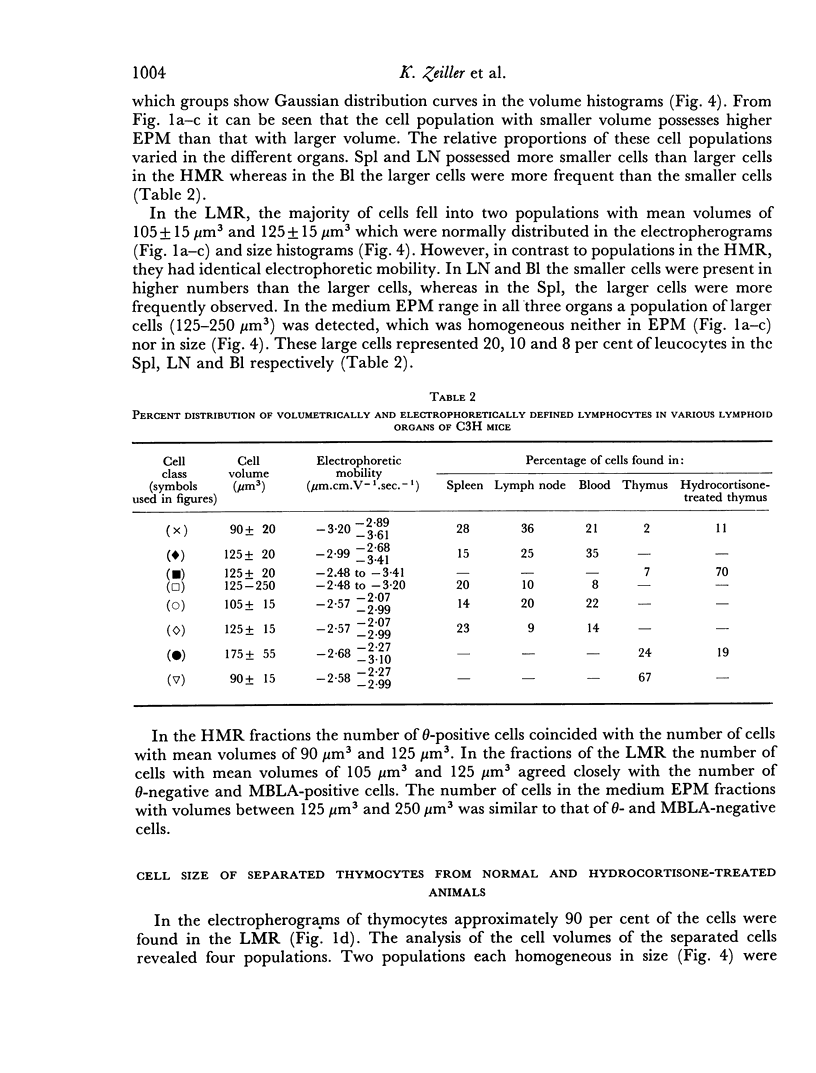
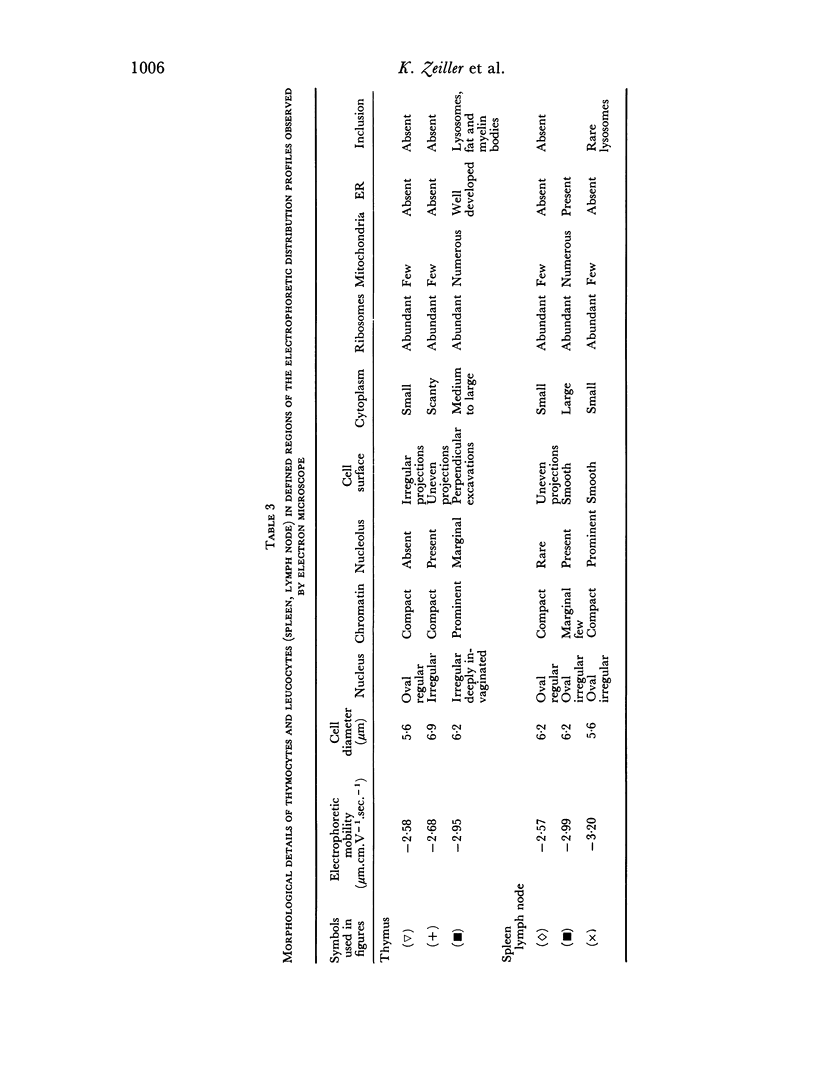
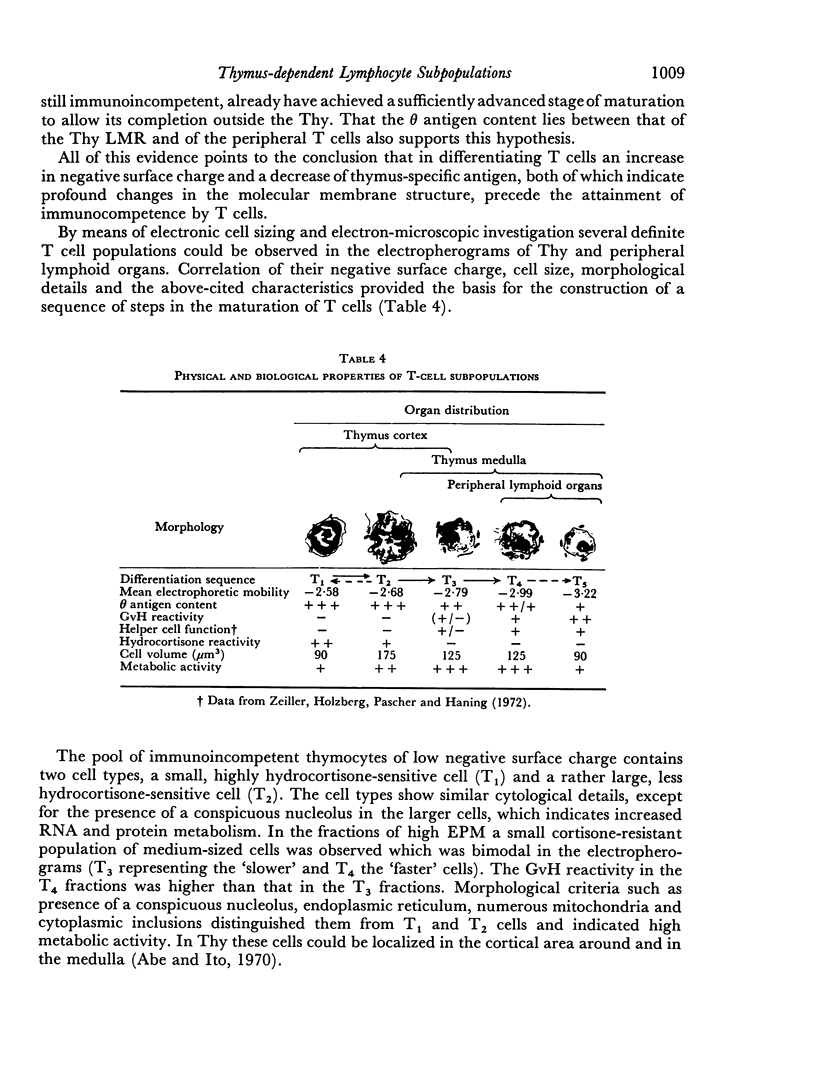
Thymus-dependent cells from thymus and peripheral lymphoid organs were preparatively separated by means of free flow electrophoresis into various subpopulations which were defined in terms of θ (theta) antigen content, negative surface charge, graft-versus-host (GvH) reactivity, hydrocortisone sensitivity, cell volume and morphological details. Most thymocytes in the cortex have a low negative surface charge, high θ antigen content, are hydrocortisone-sensitive and immuno-incompetent. On the basis of electronic cell sizing this group consists of a large population of 90 μm3 cells (T1) and a small population of 175 μm3 cells (T2), the latter being less hydrocortisone-sensitive than the former.
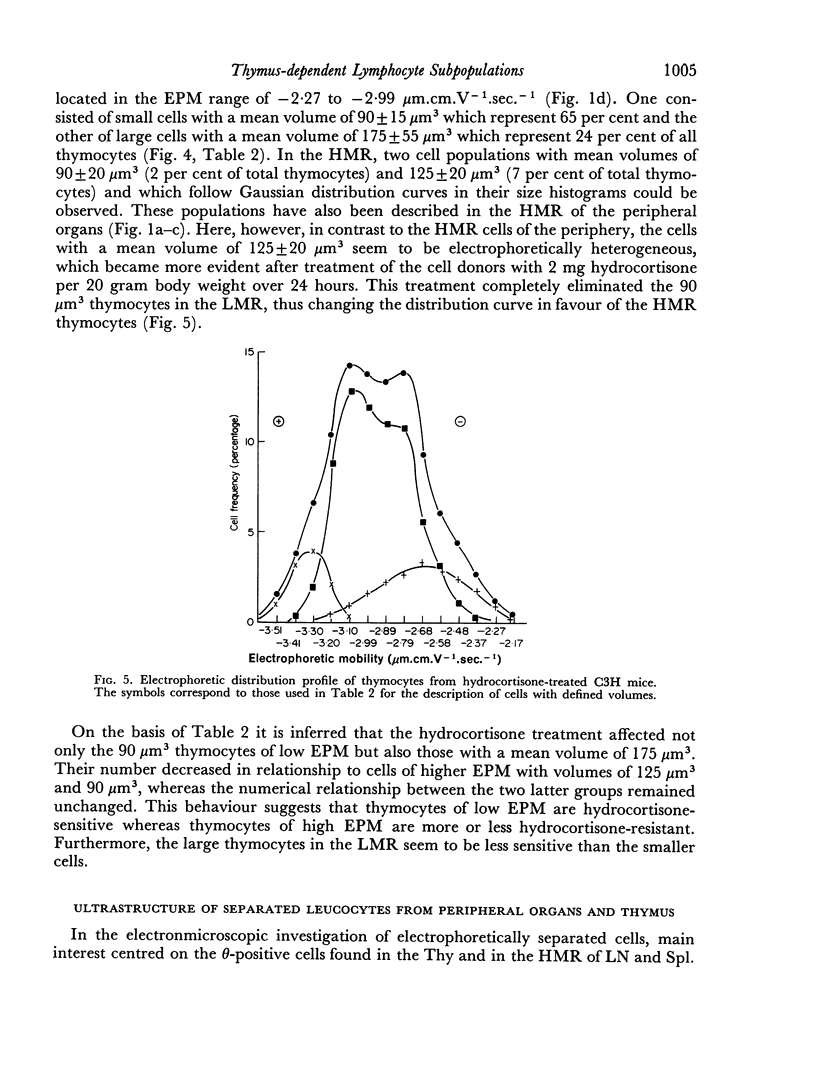
A minority of thymocytes resides in and around the medulla and has high negative surface charge, a medium θ antigen content, is hydrocortisone-resistant and reveals low GvH reactivity. These cells are medium sized (125 μm3), electrophoretically bimodal (T3 had a medium and T4 a high negative surface charge) and on the basis of morphological criteria are metabolically more active than the thymocytes of low negative surface charge.
In the peripheral lymphoid organs, all thymus-dependent cells show high negative surface charge and have the lowest observed θ antigen content and the highest observed GvH reactivity. These cells fall into two populations of which one is 125 μm3 with lower negative surface charge and the other is 90 μm3 with a somewhat higher negative surface charge. These 125 μm3 cells (T4), which morphologically resemble the 125 μm3 thymocytes, are less GvH-reactive than the 90 μm3 (T5) cells, which seem to be resting cells.
On the basis of these data, a possible sequence of steps in the maturation of T cells was constructed as follows: in the cortex of the thymus T1 thymocytes are transformed into T2 and these develop into T3 and T4 thymocytes which have higher negative surface charge, lower θ antigen content and are in an advanced stage of maturity. After further loss of θ antigen these cells, which are in the medulla, emigrate into the periphery and are finally transformed into highly immunocompetent T5 cells possessing the highest observed negative surface charge.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Ito T. Fine structure of small lymphocytes in the thymus of the mouse: qualitative and quantitative analysis by electron microscopy. Z Zellforsch Mikrosk Anat. 1970;110(3):321–335. doi: 10.1007/BF00321145. [DOI] [PubMed] [Google Scholar]
- Andersson B., Blomgren H. Evidence for a small pool of immunocompetent cells in the mouse thymus. Its role in the humoral antibody response against sheep erythrocytes, bovine serum albumin, ovalbumin and the NIP determinant. Cell Immunol. 1970 Oct;1(4):362–371. doi: 10.1016/0008-8749(70)90014-6. [DOI] [PubMed] [Google Scholar]
- Bhattacharya C. G. A simple method of resolution of a distribution into gaussian components. Biometrics. 1967 Mar;23(1):115–135. [PubMed] [Google Scholar]
- Blackburn W. R., Miller J. F. Electron microscopic studies of thymus graft regeneration and rejection. I. Syngeneic grafts. Lab Invest. 1967 Jan;16(1):66–83. [PubMed] [Google Scholar]
- Blomgren H., Andersson B. Characteristics of the immunocompetent cells in the mouse thymus: cell population changes during cortisone-induced atrophy and subsequent regeneration. Cell Immunol. 1970 Nov;1(5):545–560. doi: 10.1016/0008-8749(70)90041-9. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Andersson B. Evidence for a small pool of immunocompetent cells in the mouse thymus. Exp Cell Res. 1969 Oct;57(2):185–192. doi: 10.1016/0014-4827(69)90140-2. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Andersson B. Recirculating lymphocytes in the mouse thymus are part of the relatively cortisone resistant cell population. Clin Exp Immunol. 1972 Feb;10(2):297–303. [PMC free article] [PubMed] [Google Scholar]
- Blomgren H., Révész L. The effect of bone marrow protection on the cellular composition of irradiated mouse thymus. Exp Cell Res. 1968 Oct;53(1):261–271. doi: 10.1016/0014-4827(68)90372-8. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Svedmyr E. In vitro stimulation of mouse thymus cells by PHA and allogeneic cells. Cell Immunol. 1971 Aug;2(4):285–299. doi: 10.1016/0008-8749(71)90063-3. [DOI] [PubMed] [Google Scholar]
- Davies A. J. The thymus and the cellular basis of immunity. Transplant Rev. 1969;1:43–91. doi: 10.1111/j.1600-065x.1969.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Dukor P., Miller J. F., House W., Allman V. Regeneration of thymus grafts. I. Histological and cytological aspects. Transplantation. 1965 Sep;3(5):639–668. doi: 10.1097/00007890-196509000-00006. [DOI] [PubMed] [Google Scholar]
- HARRIS J. E., FORD C. E. CELLULAR TRAFFIC OF THE THYMUS: EXPERIMENTS WITH CHROMOSOME MARKERS. EVIDENCE THAT THE THYMUS PLAYS AN INSTRUCTIONAL PART. Nature. 1964 Feb 29;201:884–885. doi: 10.1038/201884a0. [DOI] [PubMed] [Google Scholar]
- Hannig K., Zeiller K. Zur Auftrennung und Charakterisierung immunkompetenter Zellen mit Hilfe der trägerfreien Ablenkungselektrophorese. Hoppe Seylers Z Physiol Chem. 1969 Apr;350(4):467–472. [PubMed] [Google Scholar]
- Hinrichsen K. Zellteilungen und Zellwanderungen im Thymus der erwachsenen Maus. Z Zellforsch Mikrosk Anat. 1965 Oct 28;68(3):427–444. [PubMed] [Google Scholar]
- Konda S., Nakao Y., Smith R. T. Immunologic properties of mouse thymus cells. Identification of T cell functions within a minor, low-density subpopulation. J Exp Med. 1972 Dec 1;136(6):1461–1477. doi: 10.1084/jem.136.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance E. M., Cooper S., Boyse E. A. Antigenic change and cell maturation in murine thymocytes. Cell Immunol. 1970 Nov;1(5):536–544. doi: 10.1016/0008-8749(70)90040-7. [DOI] [PubMed] [Google Scholar]
- Leckband E., Boyse E. A. Immunocompetent cells among mouse thymocytes: a minor population. Science. 1971 Jun 18;172(3989):1258–1260. doi: 10.1126/science.172.3989.1258. [DOI] [PubMed] [Google Scholar]
- Mandel T., Russell P. J. Differentation of foetal mouse thymus. Ultrastructure of organ cultures and of subcapsular grafts. Immunology. 1971 Oct;21(4):659–674. [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Basten A., Sprent J., Cheers C. Interaction between lymphocytes in immune responses. Cell Immunol. 1971 Oct;2(5):469–495. doi: 10.1016/0008-8749(71)90057-8. [DOI] [PubMed] [Google Scholar]
- Mosier D., Cantor H. Functional maturation of mouse thymic lymphocytes. Eur J Immunol. 1971 Dec;1(6):459–461. doi: 10.1002/eji.1830010610. [DOI] [PubMed] [Google Scholar]
- NOSSAL G. J. STUDIES ON THE RATE OF SEEDING OF LYMPHOCYTES FROM THE INTACT GUINEA PIG THYMUS. Ann N Y Acad Sci. 1964 Nov 30;120:171–181. doi: 10.1111/j.1749-6632.1964.tb34715.x. [DOI] [PubMed] [Google Scholar]
- Nordling S., Andersson L. C., Häyry P. Separation of T and B lymphocytes by preparative cell electrophoresis. Eur J Immunol. 1972 Oct;2(5):405–410. doi: 10.1002/eji.1830020504. [DOI] [PubMed] [Google Scholar]
- OSOBA D., MILLER J. F. EVIDENCE FOR A HUMORAL THYMUS FACTOR RESPONSIBLE FOR THE MATURATION OF IMMUNOLOGICAL FACULTY. Nature. 1963 Aug 17;199:653–654. doi: 10.1038/199653a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Nase S., Mitchison N. A. Mouse specific bone marrow-derived lymphocyte antigen as a marker for thymus-independent lymphocytes. Nature. 1971 Mar 5;230(5288):50–51. doi: 10.1038/230050a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Wortis H. H. Thymus dependence of theta-bearing cells in the peripheral lymphoid tissues of mice. Immunology. 1970 Jun;18(6):931–942. [PMC free article] [PubMed] [Google Scholar]
- Raff M. Evidence for subpopulation of mature lymphocytes within mouse thymus. Nat New Biol. 1971 Feb 10;229(6):182–184. doi: 10.1038/newbio229182a0. [DOI] [PubMed] [Google Scholar]
- Raff M. Theta isoantigen as a marker of thymus-derived lymphocytes in mice. Nature. 1969 Oct 25;224(5217):378–379. doi: 10.1038/224378a0. [DOI] [PubMed] [Google Scholar]
- SAINTE-MARIE G., LEBLOND C. P. CYTOLOGIC FEATURES AND CELLULAR MIGRATION IN THE CORTEX AND MEDULLA OF THYMUS IN THE YOUNG ADULT RAT. Blood. 1964 Mar;23:275–299. [PubMed] [Google Scholar]
- Sainte-Marie G., Leblond C. P. Elaboration of a model for the formation of lymphocytes in the thymic cortex of young adult rats. Blood. 1965 Dec;26(6):765–783. [PubMed] [Google Scholar]
- Schlesinger M., Golakai V. K. Loss of thymus-distinctive serological characteristics in mice under certain conditions. Science. 1967 Mar 3;155(3766):1114–1116. doi: 10.1126/science.155.3766.1114. [DOI] [PubMed] [Google Scholar]
- Stutman O., Yunis E. J., Good R. A. Studies on thymus function. 3. Duration of thymic function. J Exp Med. 1972 Feb 1;135(2):339–356. doi: 10.1084/jem.135.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi T., Adler W. H., Smith R. T. Identification of mouse thymus antigen recognition function in a minor, low-density, low-theta cell subpopulation. Cell Immunol. 1971 Aug;2(4):373–380. doi: 10.1016/0008-8749(71)90072-4. [DOI] [PubMed] [Google Scholar]
- Thom R., Hampe A., Sauerbrey G. Die elektronische Volumenbestimmung von Blutkörperchen und ihre Fehlerquellen. Z Gesamte Exp Med. 1969 Dec 31;151(4):331–349. [PubMed] [Google Scholar]
- Thom R., Kachel V. Fortschritte für die elektronische Grössenbestimmung von Blutkörperchen. Blut. 1970 Jul;21(1):48–50. doi: 10.1007/BF01633230. [DOI] [PubMed] [Google Scholar]
- WARNER N. L. THE IMMUNOLOGICAL ROLE OF DIFFERENT LYMPHOID ORGANS IN THE CHICKEN. II. THE IMMUNOLOGICAL COMPETENCE OF THYMIC CELL SUSPENSIONS. Aust J Exp Biol Med Sci. 1964 Jun;42:401–416. doi: 10.1038/icb.1964.38. [DOI] [PubMed] [Google Scholar]
- Zeiller K., Dolan L. Thymus specific antigen on electrophoretically separated rat lymphocytes. Tracing of the differentiation pathway of bone marrow-derived thymocytes by use of a surface marker. Eur J Immunol. 1972 Oct;2(5):439–444. doi: 10.1002/eji.1830020511. [DOI] [PubMed] [Google Scholar]
- Zeiller K., Hannig K. Free-flow electrophoretic separation of lymphocytes. Evidence for specific organ distributions of lymphoid cells. Hoppe Seylers Z Physiol Chem. 1971 Aug;352(8):1162–1167. doi: 10.1515/bchm2.1971.352.2.1162. [DOI] [PubMed] [Google Scholar]
- Zeiller K., Hannig K., Pascher G. Free-flow electrophoretic separation of lymphocytes. Separation of graft versus host reactive lymphocytes of rat spleens. Hoppe Seylers Z Physiol Chem. 1971 Aug;352(8):1168–1170. [PubMed] [Google Scholar]
- Zeiller K., Holzberg E., Pascher G., Hannig K. Free flow electrophoretic separation of T and B lymphocytes. Evidence for various subpopulations of B cells. Hoppe Seylers Z Physiol Chem. 1972 Jan;353(1):105–110. [PubMed] [Google Scholar]
- Zeiller K., Liebich H. G., Hannig K. Free-flow electrophoretic separation of lymphocytes. Two thoracic duct lymphocyte subpopulations studied after prolonged cannulation and immunization. Eur J Immunol. 1971 Nov;1(5):315–322. doi: 10.1002/eji.1830010503. [DOI] [PubMed] [Google Scholar]
- Zeiller K., Pascher G. Detection of T and B cell-specific heteroantigens on electrophoretically separated lymphocytes of the mouse. Eur J Immunol. 1973 Oct;3(10):614–618. doi: 10.1002/eji.1830031004. [DOI] [PubMed] [Google Scholar]


