Abstract
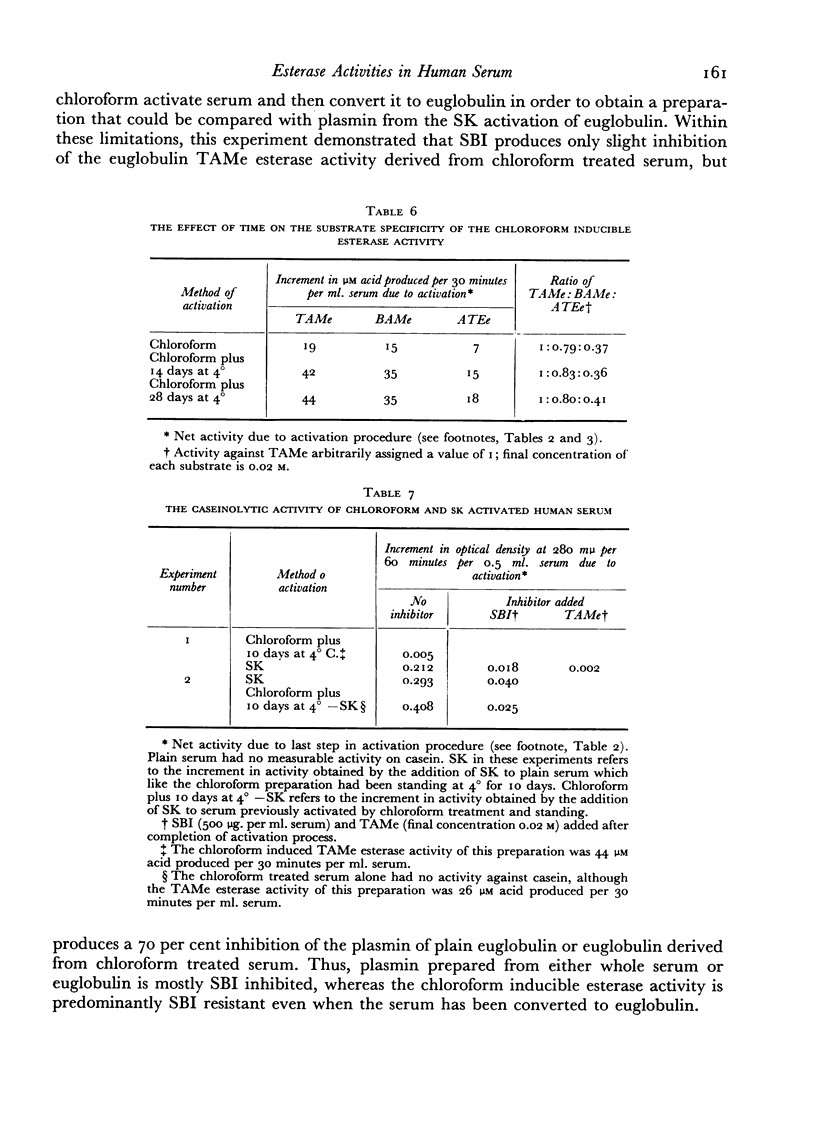
A comparative study which differentiates the chloroform inducible esterase activity of human serum from plasmin is presented. Although both esterase activities have a similar pH optimum against p-toluene sulfonyl L-arginine methyl ester, they differ in, at least, six other respects. The proenzyme of the chloroform inducible esterase activity is destroyed by heating at 56° for 30 minutes or by reducing the pH to 2.3, whereas plasminogen is stable to such treatment. The chloroform inducible esterase activity is resistant to soybean trypsin inhibitor while plasmin is mostly inhibited. Activity against N-acetyl L-tyrosine ethyl ester is induced by chloroform, but plasmin is inactive against this substrate. On the other hand, plasmin splits casein but the chloroform preparation does not. Pretreatment of serum with an antigen-antibody precipitate greatly diminishes the chloroform inducible esterase activity but not the streptokinase inducible activity.
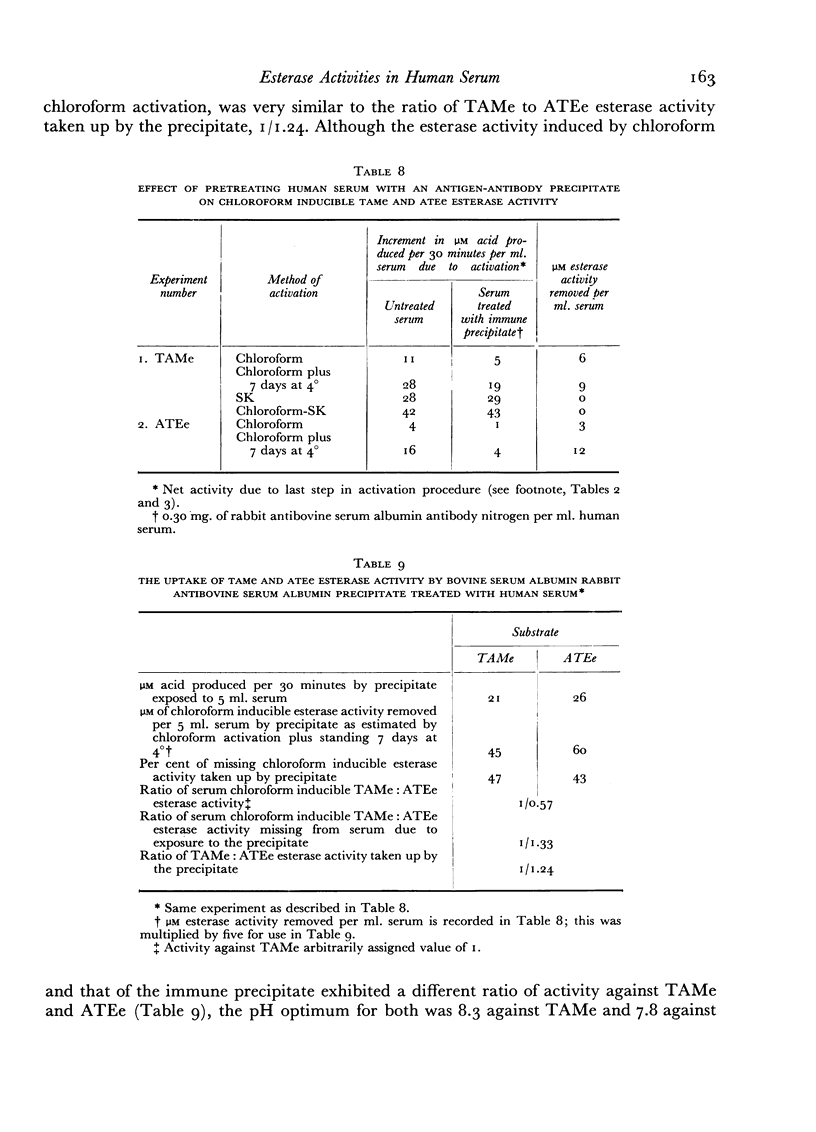
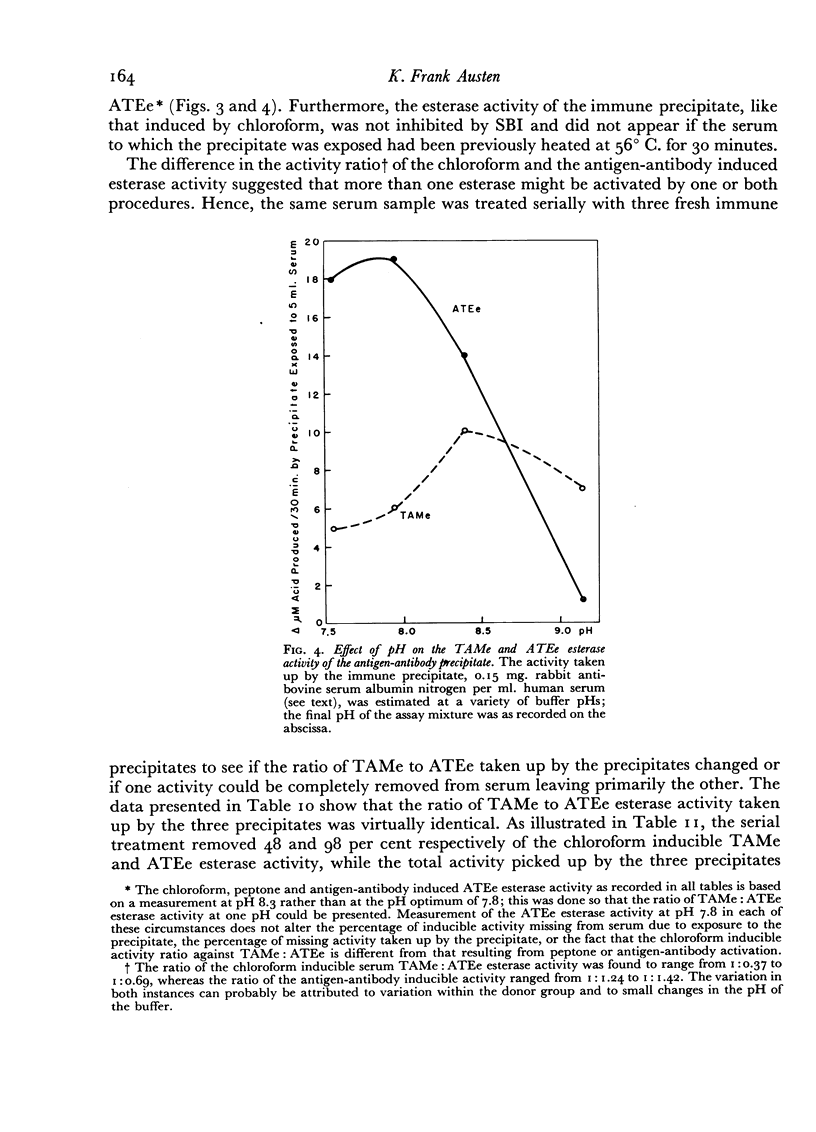
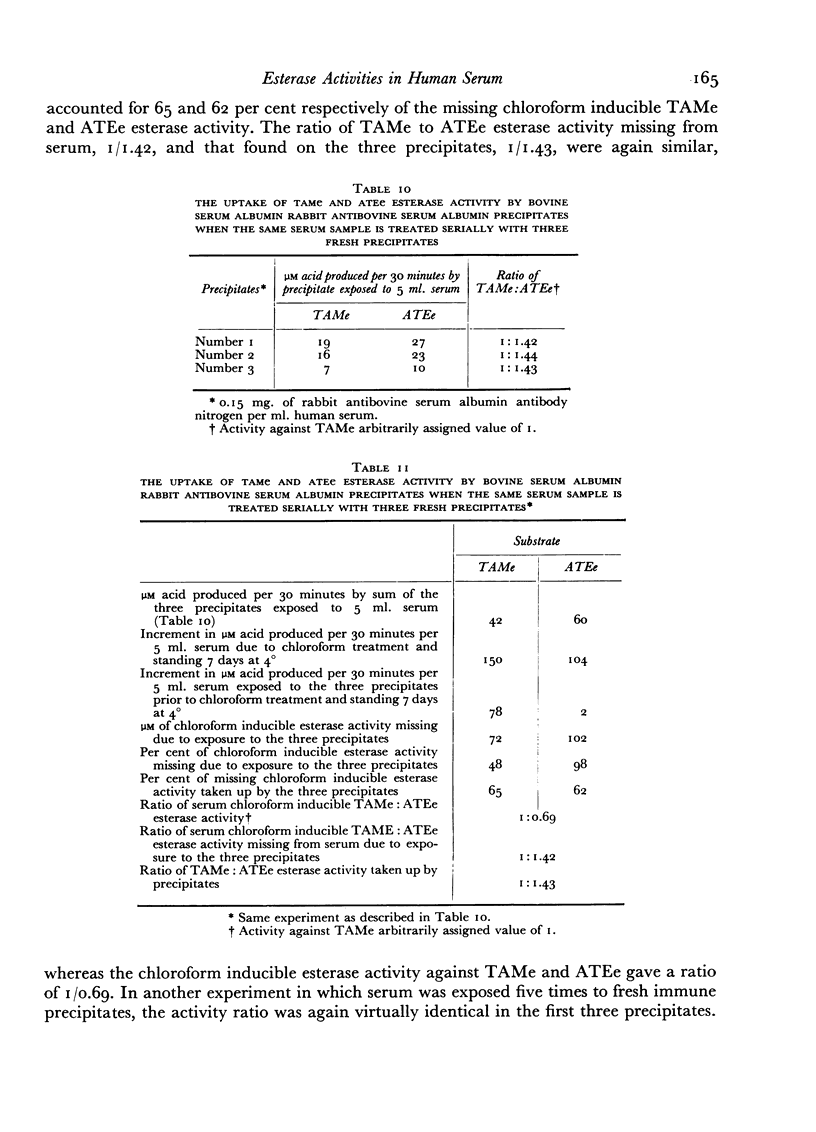
Evidence is presented which suggests that the chloroform inducible esterase activity consists of two components: one is predominantly active against p-toluene sulfonyl L-arginine methyl ester and is probably not affected by the immune precipitate; the other is more active against N-acetyl L-tyrosine ethyl ester than against p-toluene sulfonyl L-arginine methyl ester, is removed by exposing serum to immune precipitate and bears a significant resemblance to the activated first component of complement.
Peptone inducible esterase activity, like that induced by chloroform, arises from a heat and acid labile precursor, is active against p-toluene sulfonyl L-arginine methyl ester and N-acetyl L-tyrosine ethyl ester, is not inhibited by soybean trypsin inhibitor, does not digest casein and is partially removed by exposing serum to the immune precipitate before peptone activation.
The esterase activity taken up from serum by the immune precipitate, like that induced by chloroform or peptone, arises from a heat labile precursor, is active against p-toluene sulfonyl L-arginine methyl ester and N-acetyl L-tyrosine ethyl ester, is not inhibited by soybean trypsin inhibitor, and does not digest casein.
In view of the evidence that chloroform, peptone and antigen-antibody activate a similar, if not identical, proesterase, which is distinct from plasminogen, the possible role of this enzymic activity in histamine release is considered.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTRUP T., OLESEN E. S. Partial purification and fibrinolytic effect of a factor in peptone. Dan Med Bull. 1957 Aug;4(5):159–160. [PubMed] [Google Scholar]
- AUSTEN K. F., BECKER E. L., MARCUS D. M. TAMe esterase activity of guinea pig serum. I. The plasmin system. Br J Exp Pathol. 1959 Oct;40:482–493. [PMC free article] [PubMed] [Google Scholar]
- BECKER E. L., AUSTEN K. F., MARCUS D. M. TAMe esterase activity of guinea-pig serum. II. Peptone inducible esterases. Br J Exp Pathol. 1959 Oct;40:494–502. [PMC free article] [PubMed] [Google Scholar]
- BECKER E. L. Concerning the mechanism of complement action. I. Inhibition of complement activity by diisopropyl fluophosphate. J Immunol. 1956 Dec;77(6):462–468. [PubMed] [Google Scholar]
- BECKER E. L. Concerning the mechanism of complement action. II. The nature of the first component of guinea pig complement. J Immunol. 1956 Dec;77(6):469–478. [PubMed] [Google Scholar]
- BERALDO W. T. Formation of bradykinin in anaphylactic and peptone shock. Am J Physiol. 1950 Nov;163(2):283–289. doi: 10.1152/ajplegacy.1950.163.2.283. [DOI] [PubMed] [Google Scholar]
- CLIFFTON E. E. Variations in the proteolytic and the antiproteolytic reactions of serum: effect of disease, trauma, x-ray, anaphylactic shock, ACTH, and cortisone. J Lab Clin Med. 1952 Jan;39(1):105–121. [PubMed] [Google Scholar]
- ELLIOTT W. E. Volumetric determination of calcium in blood serum. J Biol Chem. 1952 May;197(2):641–644. [PubMed] [Google Scholar]
- HUMPHREY J. H., JAQUES R. The release of histamine and 5-hydroxytryptamine (serotonin) from platelets by antigen-antibody reactions (in vitro). J Physiol. 1955 Apr 28;128(1):9–27. doi: 10.1113/jphysiol.1955.sp005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEPOW I. H., PILLEMER L., RATNOFF O. D. The influence of calcium ions on the inactivation of human complement and its components by plasmin. J Exp Med. 1953 Sep;98(3):277–289. doi: 10.1084/jem.98.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEPOW I. H., WURZ L., RATNOFF O. D., PILLEMER L. Studies on the mechanism of inactivation of human complement by plasmin and by antigen-antibody aggregates. I. The requirement for a factor resembling C'1 and the role of Ca++. J Immunol. 1954 Sep;73(3):146–158. [PubMed] [Google Scholar]
- LOWELL F. C., FRANKLIN W., SCHILLER I. W., FOLLENSBY E. M. Acute allergic reactions induced in subjects with hay fever and asthma by the intravenous administration of allergens with observations on blood clot lysis. J Allergy. 1956 Jul;27(4):369–376. doi: 10.1016/0021-8707(56)90076-4. [DOI] [PubMed] [Google Scholar]
- MEYERS W. M., BURDON K. L., RILEY M. N. Proteolytic activity of whole human serum following surgery. J Lab Clin Med. 1957 Mar;49(3):377–385. [PubMed] [Google Scholar]
- NORMAN P. S. Studies of the plasmin system. I. Measurement of human and animal plasminogen; measurement of an activator in human serum. J Exp Med. 1957 Sep 1;106(3):423–437. doi: 10.1084/jem.106.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PILLEMER L., RATNOFF O. D., BLUM L., LEPOW I. H. The inactivation of complement and its components by plasmin. J Exp Med. 1953 Apr;97(4):573–589. doi: 10.1084/jem.97.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATNOFF O. D., LEPOW I. H. Some properties of an esterase derived from preparations of the first component of complement. J Exp Med. 1957 Aug 1;106(2):327–343. doi: 10.1084/jem.106.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERRY S., FLETCHER A. P., ALKJAERSIG N. Fibrinolysis and fibrinolytic activity in man. Physiol Rev. 1959 Apr;39(2):343–382. doi: 10.1152/physrev.1959.39.2.343. [DOI] [PubMed] [Google Scholar]
- Silva M. R., Rimington C. Studies on the activation and purification of blood fibrinolysin. Biochem J. 1948;43(2):163–168. [PMC free article] [PubMed] [Google Scholar]
- TROLL W., SHERRY S., WACHMAN J. The action of plasmin on synthetic substrates. J Biol Chem. 1954 May;208(1):85–93. [PubMed] [Google Scholar]
- Tagnon H. J., Davidson C. S., Taylor F. H. STUDIES ON BLOOD COAGULATION: A PROTEOLYTIC ENZYME PREPARED FROM CALCIUM AND PLATELET FREE NORMAL HUMAN BLOOD PLASMA. J Clin Invest. 1942 Sep;21(5):525–531. doi: 10.1172/JCI101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNGAR G., DAMGAARD E., HUMMEL F. P. Activation of profibrinolysin by antigen-antibody reaction and by anaphylactoid agents; its relation to complement. J Exp Med. 1953 Oct;98(4):291–303. doi: 10.1084/jem.98.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]


