Fig. 8.
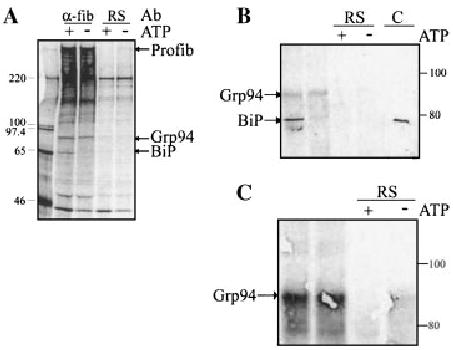
Identification of proteins associated with profibrillin-1 in the secretory pathway. A: Neonatal dermal fibroblasts were metabolically labeled with [35S]cysteine for 4 h, lysed in PBS–TDS, and immunoprecipitated with either anti-fibrillin-1 antibody (α-fib) or rabbit serum (RS) as a negative control. The migrations of proteins consistently found associated with profibrillin-1 are indicated. B: Neonatal dermal fibroblasts were lysed in ATP-depleting buffer, and immunoprecipitated with either anti-fibrillin-1 antibody or pre-immune RS as a negative control. Lysates were then subjected to incubation with or without ATP (+, −). After electrophoresis, immunoblot analysis of immunoprecipitated CL confirmed the identification of BiP using a KDEL-specific monoclonal antibody, which recognized a protein of the correct migration in both the fibroblast cell lysate and the positive control (C). This antibody also recognized a protein at the expected migration for Grp94. C: Immunoblot of immunoprecipitated CL after incubation with or without ATP (+, −). The identity of Grp94 was confirmed using an anti-Grp94-specific antibody.
