Fig. 2.
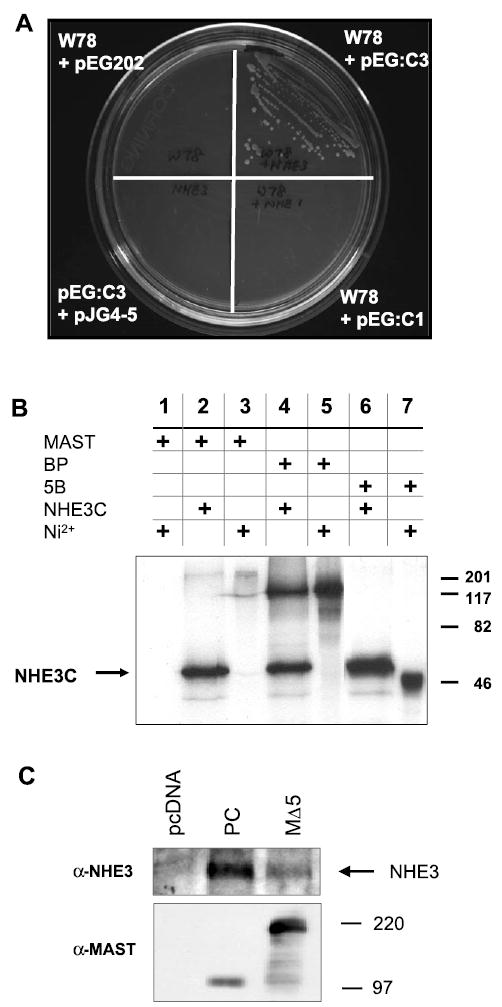
MAST205 interacts with Na+/H+ exchanger (NHE) 3. A: interaction of W78 with NHE3C in yeast. Yeast strain EGY48 was transformed with W78 with or without pEG:C3 or pEG;C1. EGY48 grew on a minimal plate lacking uracil, histidine, and leucine only in the presence of both W78 and pEG:C3 but not in the presence of pEG:C1 and W78. W78 + pEG202 or pEG:C3 + pJG4–5 did not revive the yeast. B: in vitro interaction between MAST205 and NHE3. The NHE3 COOH-terminal domain (NHE3C) was synthesized as 6xHis proteins and labeled with [35S]Met. As described in methods and materials, NHE3C immobilized on Ni2+-nitrilotriacetic acid (Ni-NTA) beads was incubated for 2 h with 20 μl [35S]Met-labeled MAST205, BP, and 5B. The protein complexes were eluded by boiling in 2 × sample buffer, resolved by SDS-PAGE, and visualized by autoradiography. The eluded protein samples were loaded in lanes 2, 4, and 6. In lanes 3, 5, and 7, 2 μl of the in vitro products were loaded to show the sizes and the relative labeling of each construct. Lane 1 shows a negative control where [35S]Met-labeled MAST205 was incubated with Ni-NTA beads without NHE3C. Similar results were obtained in three experiments. C: opossum kidney (OK) cells were transfected with pcDNA3.1-HisB, PC, or MΔ5. Transfected cells were lysed, and the expressed MΔ5 and PC proteins were affinity purified with Ni-NTA beads. Top: the presence of copurified NHE3 was detected by Western immunoblotting using a monoclonal anti-NHE3 antibody. Bottom: Western immunoblot using anti-MAST205 antiserum on lysates from transfected cells. Similar results were obtained in three experiments.
