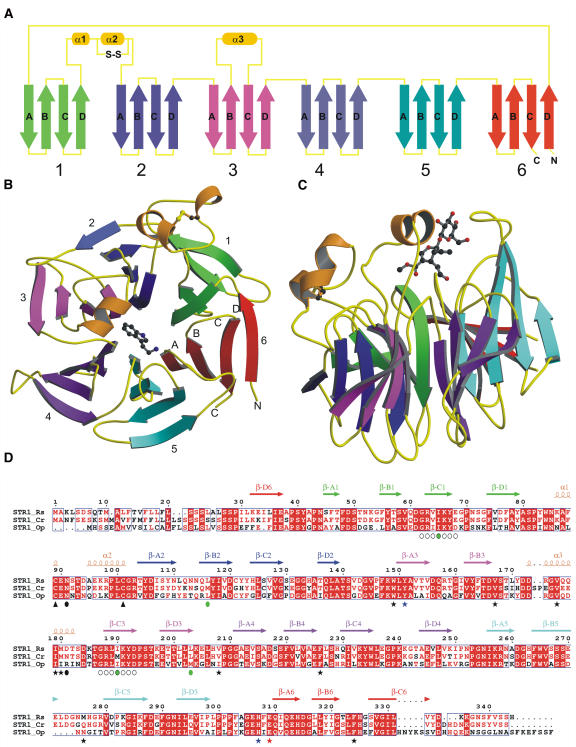
Figure 2.
Overview of the R. serpentina STR1 Structure.
(A) The topology of the STR1 structure. Each blade (consisting of four β-strands) of the propeller is shown in various colors (green, blue, magenta, purple, cyan, and red), and the connecting loop is shown in yellow and helices in orange.
(B) Front view of the six-bladed β-propeller in complex with tryptamine. The top of the propeller is, by convention, the face carrying the loops connecting the β-B strand and β-C strand in each blade.
(C) Side view of the propeller in complex with secologanin.
(D) Sequence alignment of STR1 from different plant species. STR1_Rs, STR1 from R. serpentina and from R. mannii; STR1_Cr, STR1 from C. roseus (sequence identity 79%); STR1_Op, STR1 from O. pumila (sequence identity 58%). Four residues that were mutated to Met are marked with green circles; internal repetitive sequences that were used to design the fourth Met mutation I65M are indicted with open circles below the sequence. Residues that form the hydrophobic active site are highlighted with black asterisks; two polar residues within the active site are marked with blue asterisks; the catalytic residue Glu-309 is marked with a red asterisk. The conserved disulfide bridge is indicated by black arrowheads below the Cys residues. Putative N-glycosylation residues are indicated by black circles.