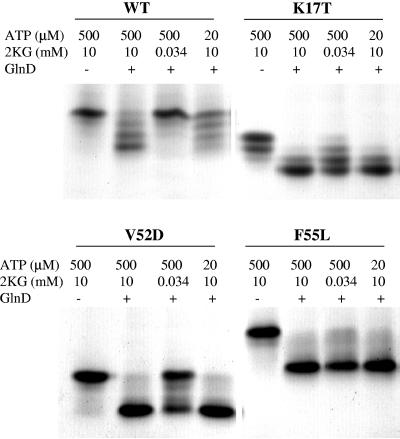
FIG. 5.
In vitro uridylyltransferase activity assays on wild-type (WT) GlnB and GlnB* variants show that many of these variants are dramatically altered in their properties as GlnD substrates. Wild-type GlnB and four GlnB* proteins were overexpressed and purified from E. coli (UQ2549, UQ4308, UQ4310, UQ4311, and UQ4312) and then used as substrates of E. coli GlnD as described in Materials and Methods. The specific GlnB protein used in each portion of the gel is shown at the top, and below that are the specific conditions for the samples in each lane. These are native gels, so that the protein trimer runs at as many as four positions, depending on the number of UMP groups per trimer, with the fully uridylylated form moving the fastest.