Abstract
1 Three healthy male volunteers were treated with benzoylmetronidazole suspension (3.2 g equivalent to 2 g metronidazole) in a pilot study to investigate the absorption of benzoylmetronidazole into the systemic circulation. A further ten healthy male volunteers took part in a crossover study to compare the pharmacokinetics of metronidazole and its principal oxidative metabolites after administration of benzoylmetronidazole (equivalent to 2 g or 400 mg of metronidazole) or metronidazole (400 mg).
2 The plasma pharmacokinetics of metronidazole and metabolite I[1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole] and plasma and urinary concentrations of these, plus benzoylmetronidazole and metabolite II [2-methyl-5-nitroimidazole-1-acetic acid], were determined using specific and sensitive high performance liquid chromatographic assay procedures.
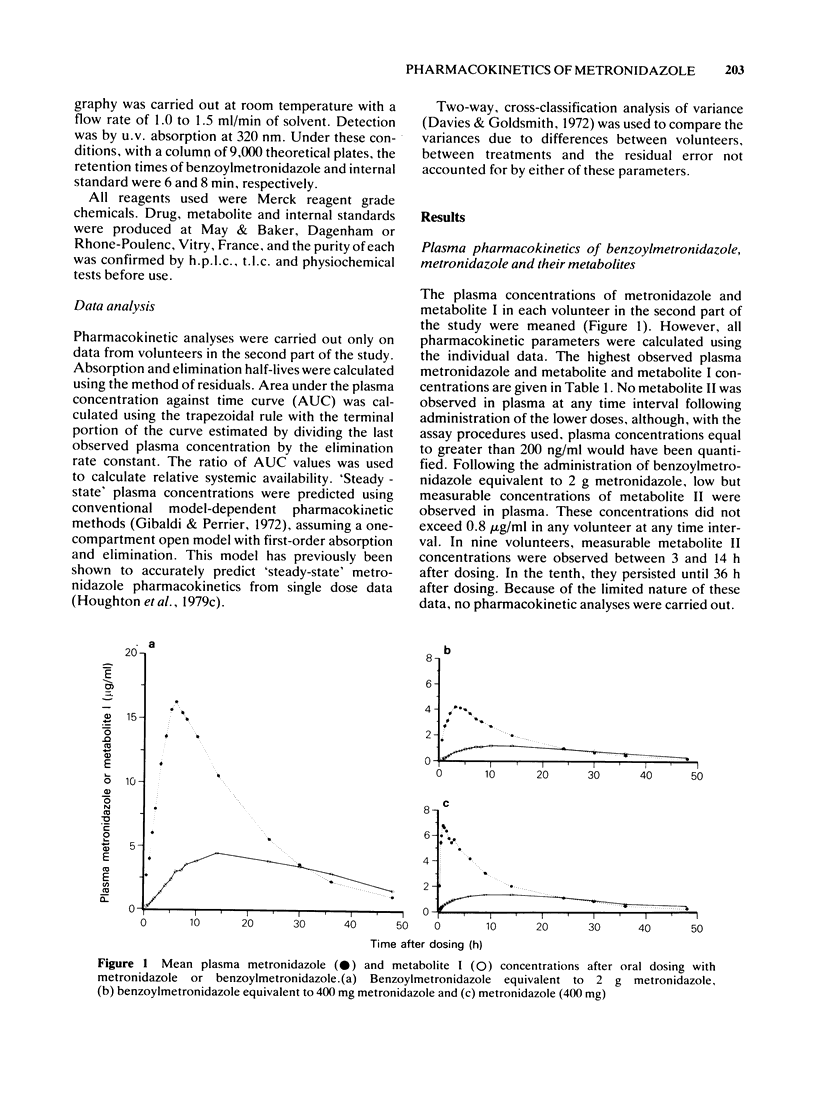
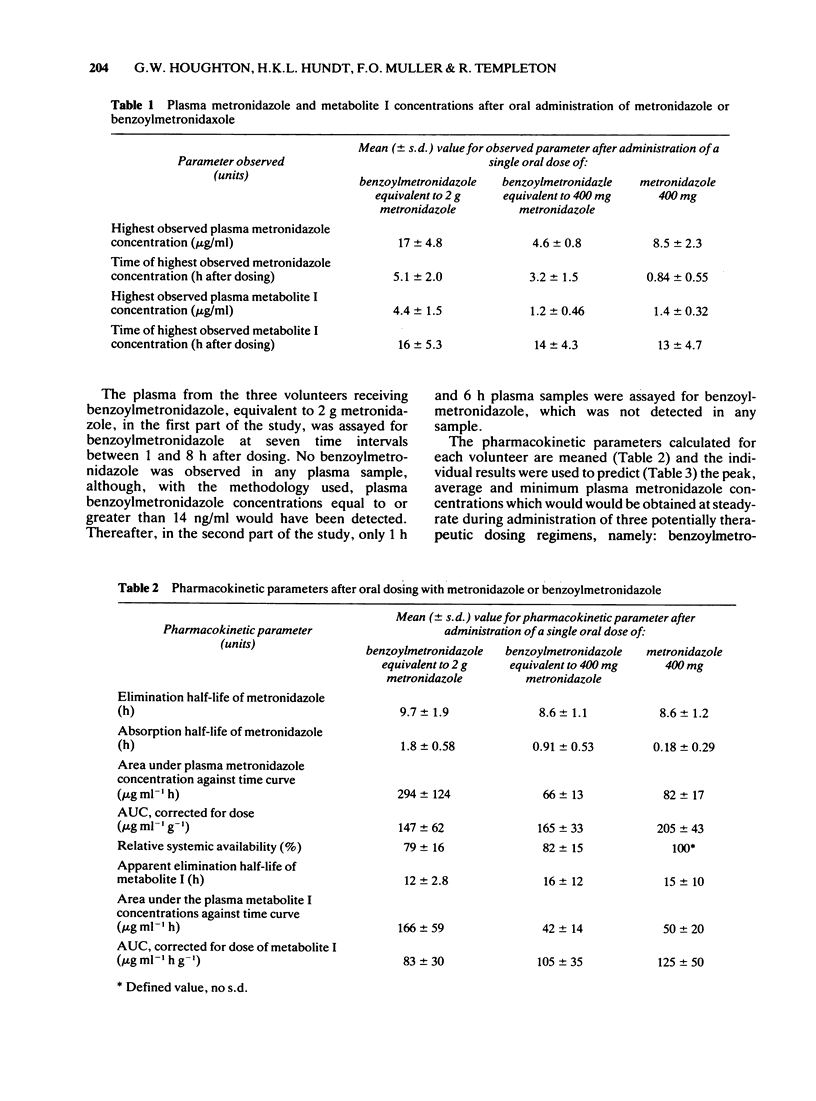
3 No benzoylmetronidazole was observed in any plasma or urine sample assayed. The values for and times of the highest observed plasma metronidazole concentrations after a single oral dose of benzoylmetronidazole, equivalent to 2 g and 400 mg metronidazole, were 17 μg/ml at 5.1 h after dosing and 4.6 μg/ml at 3.2 h after dosing, respectively. Following oral administration of metronidazole (400 mg), the comparable values were 8.5 μg/ml at 0.8 h after dosing. Peak plasma concentrations of metabolite I after each dose were comparable with each other when corrected for the amount of metronidazole reaching the systemic circulation. The peak concentrations of this metabolite were markedly lower than the peak metronidazole concentrations in the same volunteer. Metabolite II was observed in low concentrations (0.8 μg/ml or less) in plasma at a few time intervals after administration of the higher dose of benzoylmetrinidazole and was not detected at any time interval after administration of benzoylmetronidazole (640 mg, equivalent to 400 mg metronidazole) or metronidazole (400 mg).
4 Pharmacokinetic parameters of metronidazole absorption are markedly different after administration of benzoylmetronidazole than after dosing with metronidazole, but the pharmacokinetic parameters of metronidazole and metabolite I elimination are essentially identical after equimolar doses of each form of the drug. The systemic availability of metronidazole derived from benzoylmetronidazole is approximately 80% of that from metronidazole and is independent of dose over the range studied.
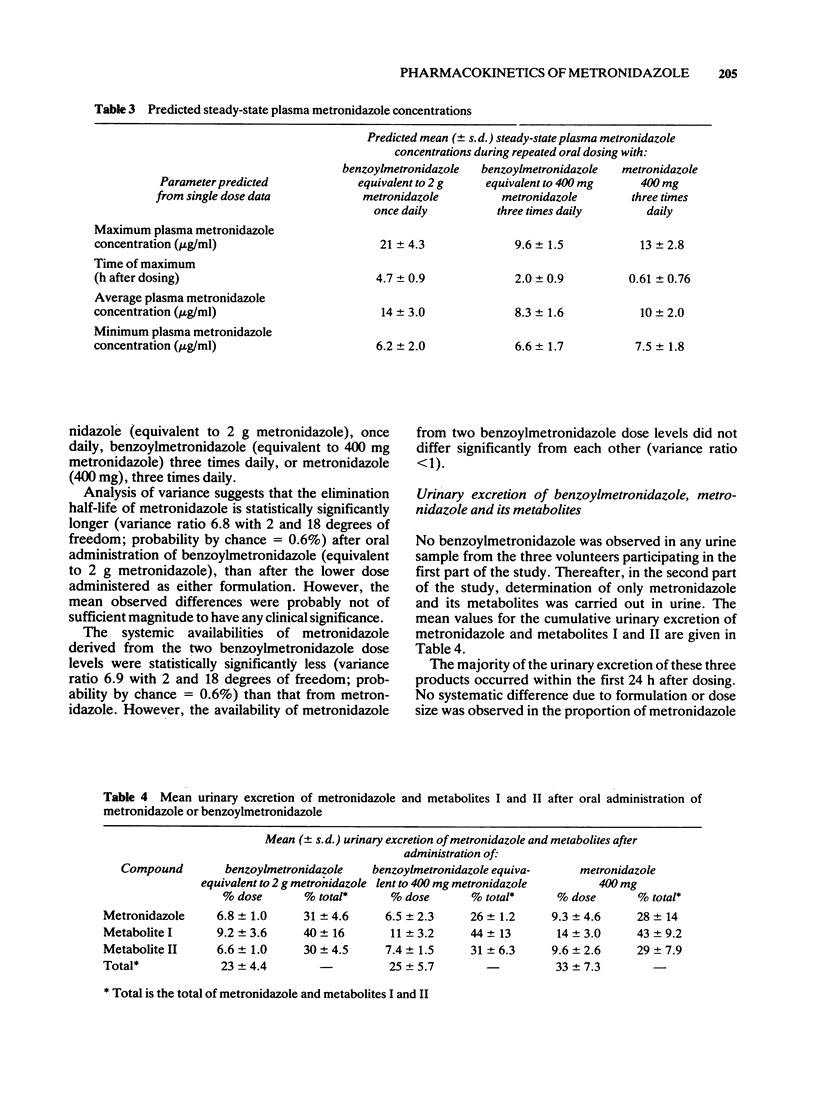
5 The mean value for minimum plasma metronidazole concentration at steady-state during the o.d. administration of benzoylmetronidazole (3.2 g equivalent to 2 g metronidazole) was predicted (from these single dose data) to be 6.2 μg/ml. Thus, these predictions suggest that the majority of patients will maintain therapeutic plasma metronidazole concentrations for the whole of the dosing interval during a once-daily dosing regimen. This oral liquid formulation of metronidazole may thus be regarded as a suitable alternative to other presentations of the drug.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Galgiani J. N., Busch D. F., Brass C., Rumans L. W., Mangels J. I., Stevens D. A. Bacteroides fragilis endocarditis, bacteremia and other infections treated with oral or intravenous metronidazole. Am J Med. 1978 Aug;65(2):284–289. doi: 10.1016/0002-9343(78)90821-5. [DOI] [PubMed] [Google Scholar]
- Gulaid A., Houghton G. W., Lewellen O. R., Smith J., Thorne P. S. Determination of metronidazole and its two major metabolites in biological fluids by high pressure liquid chromatography. Br J Clin Pharmacol. 1978 Nov;6(5):430–432. doi: 10.1111/j.1365-2125.1978.tb04608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton G. W., Smith J., Thorne P. S., Templeton R. The pharmacokinetics of oral and intravenous metronidazole in man. J Antimicrob Chemother. 1979 Sep;5(5):621–623. doi: 10.1093/jac/5.5.621. [DOI] [PubMed] [Google Scholar]
- Houghton G. W., Thorne P. S., Smith J., Templeton R., Collier J. Comparison of the pharmacokinetics of metronidazole in healthy female volunteers following either a single oral or intravenous dose. Br J Clin Pharmacol. 1979 Oct;8(4):337–341. doi: 10.1111/j.1365-2125.1979.tb04715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham H. R., Rich G. E., Selkon J. B., Hale J. H., Roxby C. M., Betty M. J., Johnson R. W., Uldall P. R. Treatment with metronidazole of three patients with serious infections due to Bacteroides fragilis. J Antimicrob Chemother. 1975 Jun;1(2):235–242. doi: 10.1093/jac/1.2.235. [DOI] [PubMed] [Google Scholar]
- Sutter V. L., Kwok Y. Y., Finegold S. M. Standardized antimicrobial disc susceptibility testing of anaerobic bacteria. I. Susceptibility of Bacteroides fragilis to tetracycline. Appl Microbiol. 1972 Feb;23(2):268–275. doi: 10.1128/am.23.2.268-275.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


