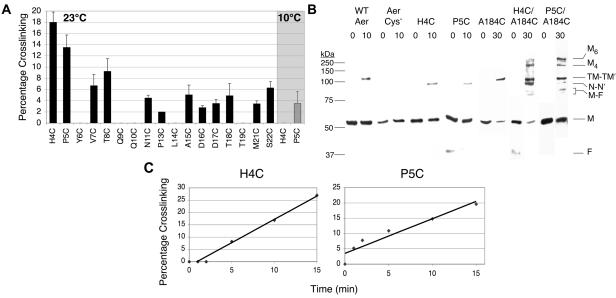
FIG. 4.
In vivo disulfide bond formation between N-cap residues of cognate Aer-PAS domains. Disulfide bond formation was determined after exposing intact cells to copper phenanthroline as described in the text. (A) Percentage of specified cysteine replacements that formed disulfide bonds in the receptorless strain after 10 min at 23°C (black bars), or after 20 min at 10°C (shaded region). Averages were derived from the results of 2 to 4 independent experiments, and error bars indicate the standard deviations from the means. (B) Western blots of cross-linked Aer proteins. Cellular proteins were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Aer was visualized by chemiluminescent Western blotting, using anti-Aer2-166 antisera. Cross-linked N-cap dimers (N-N′) were approximately 10-kDa smaller than cross-linked WT Aer (which cross-links at C253 and C203) or Aer-A184C (TM-TM′). The double-cysteine mutants Aer-H4C/A184C and Aer-P5C/A184C cross-linked to form higher-order products, which may represent tetramers (M4) and hexamers (M6) of Aer. A 37-kDa proteolytic fragment of Aer (F) was often present (38); this fragment dimerized with a full-length Aer monomer (M) to yield two faint bands (M-F) generated from single disulfide bonds at A184C or an N-Cap cysteine. Protein standards are on the left (Precision Plus, Bio-Rad). (C) Rates of Aer-H4C and Aer-P5C cross-linking over 15 min. After determining that a 10-min exposure to oxidant was in the linear range for disulfide formation, we subsequently used 10 min for fixed-time assays at 23°C.