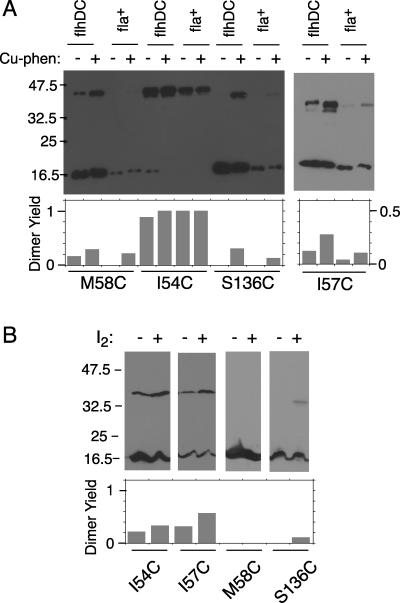
FIG. 4.
Efficient cross-linking by four Cys replacements on the FliN domain of known structure. (A) Cross-linking by Cu[1,10-phenanthroline]3. The experiments used either the fliN-null strain DFB223 or the flhDC strain RP3098, as indicated. DFB223 is flagellate when it expresses the FliN protein from the plasmid, whereas strain RP3098 is nonflagellate, and so the FliN is not present in flagellar motors. Experiments used either a 10% gel (57C) or 12% gels (58C, 54C, and 136C). Products of cross-linking, here and in panel B, are visualized on anti-FliN immunoblots. (B) Cross-linking of the same proteins by iodine. The experiment used the fliN-null strain DFB223 and 10% polyacrylamide gels. Iodine was used at 0.4 mM for the 54C and 57C proteins and 0.2 mM for the 58C and 136C proteins. (These were chosen as those showing the highest yield, in a series of experiments using 0.2, 0.4, or 0.6 mM.)