FIG. 1.
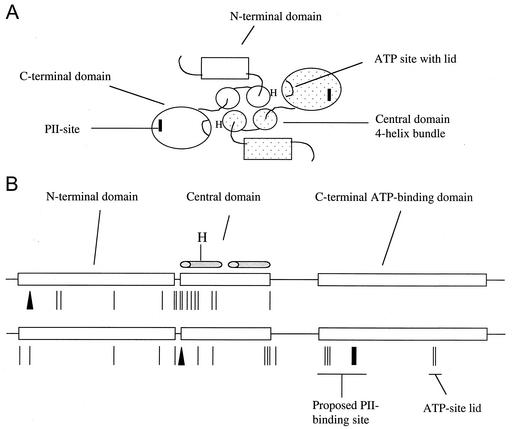
Mutations affecting the phosphatase activity of NRII map to all domains of the protein. (A) Model for the domain organization of the NRII dimer based on structural information available for other two-component system transmitter proteins and biochemical studies of NRII. Each NRII subunit is composed of three domains: an unconserved N-terminal domain involved in intramolecular signal transduction, and central and C-terminal domains that together compose the conserved transmitter module. The central domain is involved in dimerization and probably forms half of a four-helix bundle containing His139, the site of autophosphorylation. The C-terminal domain is the ATP-binding kinase domain that directly interacts with PII. The dimer is shown viewed down the four-helix bundle. (B) Linear depiction of the domain structure of the 349-amino-acid NRII showing the distribution of mutations affecting the phosphatase activity. The top drawing shows the mutations obtained in the 1992 study (5), while the bottom drawing shows the mutations obtained in this study. The lines indicate amino acid substitutions, the bar indicates a deletion, and the triangles indicate insertions. The figure is roughly to scale.