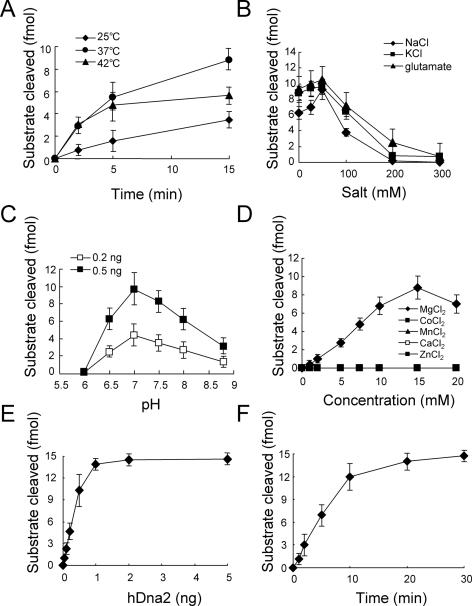
Figure 2.
Determination of biochemical properties of endonuclease activity of hDna2. (A) Determination of an optimal temperature for endonuclease activity of hDna2. The reaction mixture (80 µl) containing 0.5 ng of hDna2 was incubated at 25, 37 or 42°C. Aliquots (20 µl) were withdrawn at the indicated times. The reaction products were analyzed by native 10% PAGE in 1× TBE as described under Materials and Methods. (B) Influence of salt on endonuclease activity of hDna2. The standard endonuclease assays were carried out as described under Materials and Methods except concentration of added salt. (C) Determination of optimal pH. The standard endonuclease assays were performed as described under Materials and Methods except pH. MES-NaOH (2-Morpholinoethanesulfonic acid) was used in pH 6.0 and pH 6.5 buffer. Tris–HCl was used in other pH buffer. (D) Requirement of divalent ions. The standard endonuclease assays were carried out as described under Materials and Methods using various divalent ions. (E) Enzyme titration. Increasing amounts of hDna2 were added to the standard reaction mixture. Nucleolytic products were analyzed under Materials and Methods. (F) Kinetic analysis of endonuclease activity of hDna2. The reaction mixture (140 µl) contained 3.5 ng of hDna2 (0.5 ng per reaction); aliquots (20 µl) were withdrawn at the indicated times and the reaction products were analyzed under Materials and Methods.