Abstract
1 The pharmacokinetic and diuretic response of frusemide have been investigated in six normal subjects. Frusemide (80 mg) was administered (a) intravenously to unstressed subjects, (b) orally to unstressed subjects, (c) orally to sodium depleted subjects who had received 80 mg oral frusemide 36 h previously followed by a 20 mmol sodium, 160 mmol potassium diet.
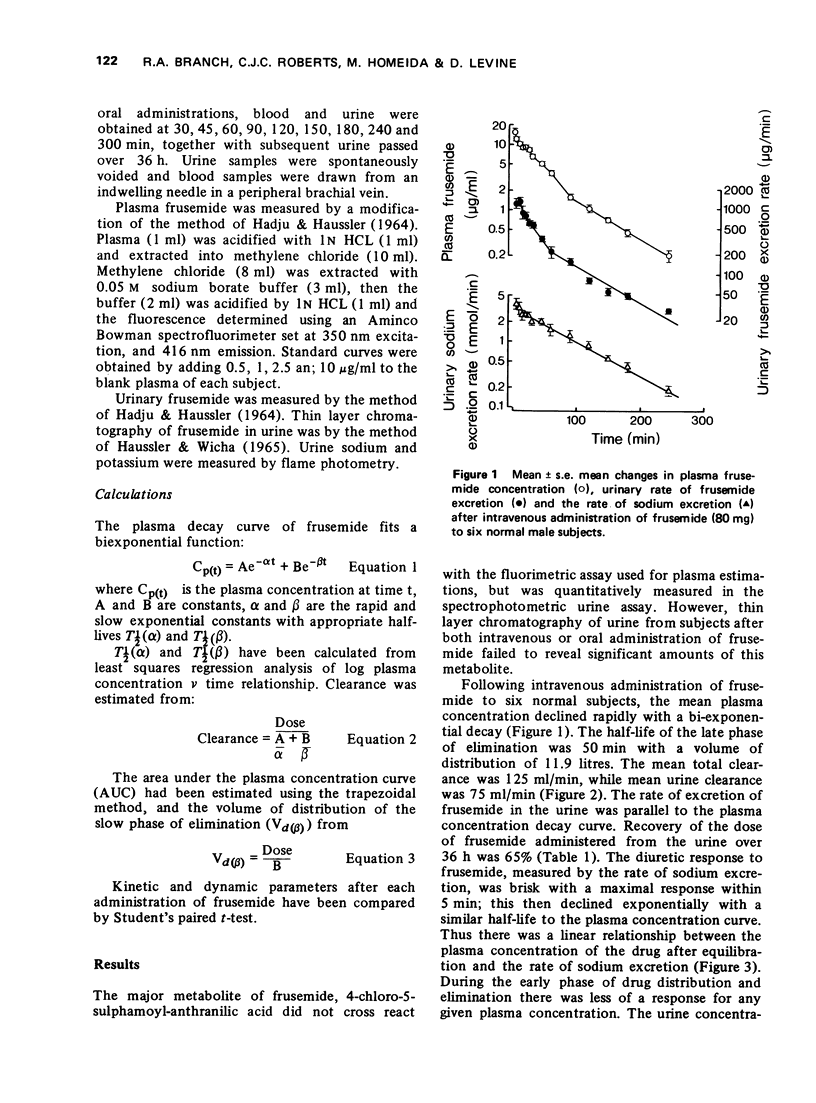
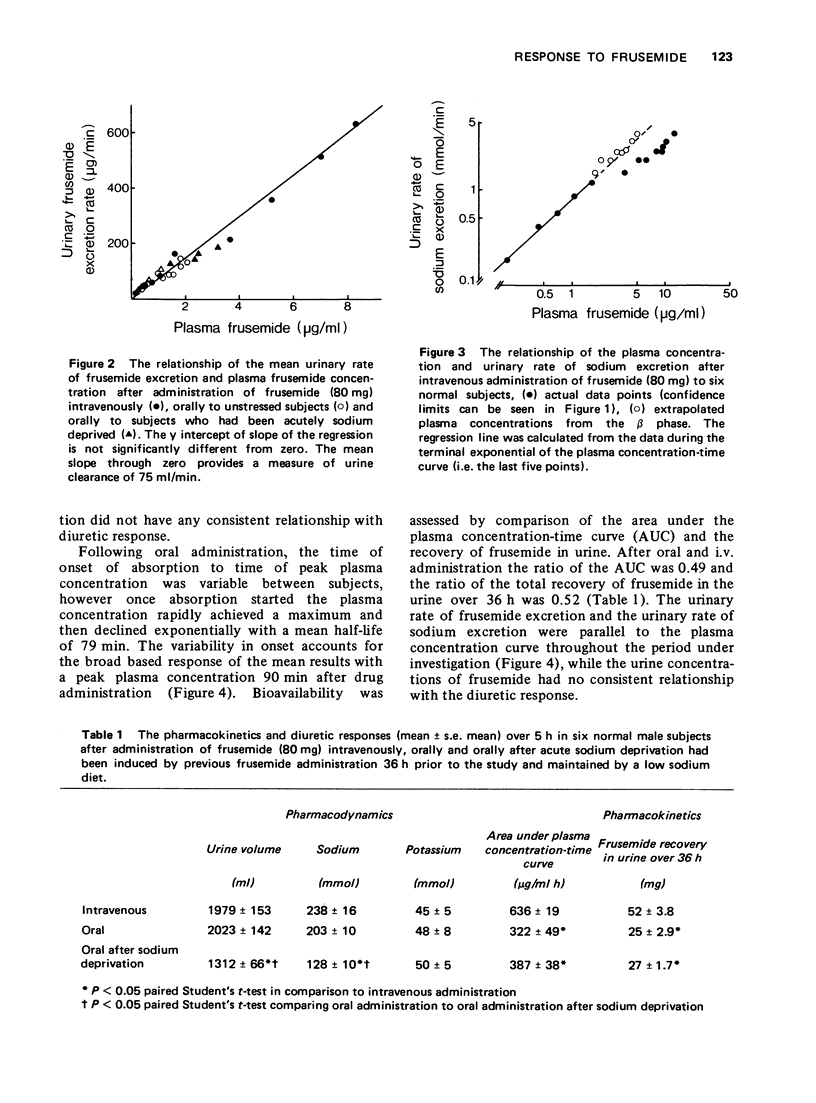
2 After i.v. administration, the logarithmic plasma concentration-time curve was biexponential. There was a linear relationship between the frusemide plasma concentration in the β-phase of elimination and the rate of sodium excretion. Urinary clearance of frusemide was 60% of total plasma clearance, similarly recovery of frusemide in the urine over 36 h was 65% of the dose administered. These observations suggested an extrarenal route of elimination.
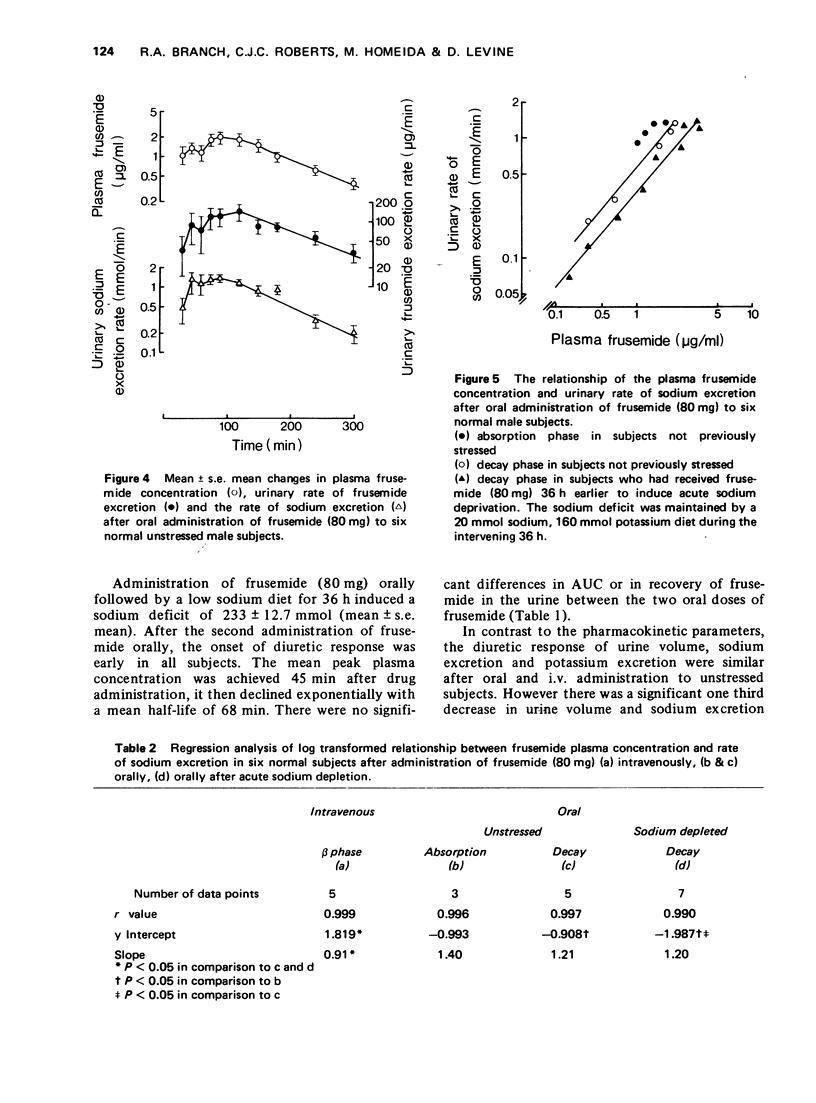
3 After oral administration there was also a linear relationship between frusemide plasma concentration and rate of sodium excretion. Oral bioavailability estimated from the ratio of the areas under the plasma concentration-time curve (AUC) and urine recovery over 36 h after i.v. and oral administration was approximately 50%, yet the diuretic response was similar.
4 The AUC of the β-phase after i.v. administration was similar to the total AUC after oral administration suggesting that response was related to drug present in a tissue pool rather than in plasma. After sodium depletion, there was no change in frusemide kinetics, however the diuretic response decreased. Once again, there was a significant relationship between plasma concentration and rate of sodium excretion. This relationship during the elimination phase after oral administration to sodium depleted subjects was significantly shifted to the right compared to the elimination phase after oral administration to unstressed subjects, suggesting a change in plasma concentration response.
5 In conclusion, the response to frusemide is determined by the concentration of drug in the tissue comparement. This response is modified by factors controlling sodium homeostasis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen F., Jakobsen P. Determination of furosemide in blood plasma and its binding to proteins in normal plasma and in plasma from patients with acute renal failure. Acta Pharmacol Toxicol (Copenh) 1974 Jul;35(1):49–57. doi: 10.1111/j.1600-0773.1974.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Beermann B., Dalén E., Lindström B., Rosén A. On the fate of furosemide in man. Eur J Clin Pharmacol. 1975 Oct 10;9(1):51–61. doi: 10.1007/BF00613429. [DOI] [PubMed] [Google Scholar]
- Calesnick B., Christensen J. A., Richter M. Absorption and excretion of furosemide-S35 in human subjects. Proc Soc Exp Biol Med. 1966 Oct;123(1):17–22. doi: 10.3181/00379727-123-31391. [DOI] [PubMed] [Google Scholar]
- Cutler R. E., Forrey A. W., Christopher T. G., Kimpel B. M. Pharmacokinetics of furosemide in normal subjects and functionally anephric patients. Clin Pharmacol Ther. 1974 Jun;15(6):588–596. doi: 10.1002/cpt1974156588. [DOI] [PubMed] [Google Scholar]
- Hook J. B., Williamson H. E. Influence of probenecid and alterations in acid-base balance of the saluretic activity of furosemide. J Pharmacol Exp Ther. 1965 Sep;149(3):404–408. [PubMed] [Google Scholar]
- Kelly M. R., Cutler R. E., Forrey A. W., Kimpel B. M. Pharmacokinetics of orally administered furosemide. Clin Pharmacol Ther. 1974 Feb;15(2):178–186. [PubMed] [Google Scholar]
- Koch-Weser J. Bioavailability of drugs (second of two parts). N Engl J Med. 1974 Sep 5;291(10):503–506. doi: 10.1056/NEJM197409052911005. [DOI] [PubMed] [Google Scholar]
- LEVY G., CALESNICK B., WASE A. RELATIONSHIP BETWEEN HG-203 EXCRETION RATE AND DIURETIC RESPONSE FOLLOWING HG-203 MERCAPTOMERIN SODIUM ADMINISTRATION. J Nucl Med. 1964 Apr;5:302–303. [PubMed] [Google Scholar]
- Nicholls M. G., Espiner E. A., Donald R. A., Hughes H. Aldosterone and its regulation during diuresis in patients with gross congestive heart failure. Clin Sci Mol Med. 1974 Oct;47(4):301–315. doi: 10.1042/cs0470301. [DOI] [PubMed] [Google Scholar]
- Rupp W. Pharmacokinetics and pharmacodynamics of Lasix. Scott Med J. 1974;19 (Suppl 1):5–13. doi: 10.1177/00369330740190S103. [DOI] [PubMed] [Google Scholar]
- Rupp W., Zapf R. M. Beispiele zur Phase I: Diuretika. Arzneimittelforschung. 1973 Nov;23(11 Suppl):1665–1668. [PubMed] [Google Scholar]
- Stason W. B., Cannon P. J., Heinemann H. O., Laragh J. H. Furosemide. A clinical evaluation of its diuretic action. Circulation. 1966 Nov;34(5):910–920. doi: 10.1161/01.cir.34.5.910. [DOI] [PubMed] [Google Scholar]
- Wagner J. G., Aghajanian G. K., Bing O. H. Correlation of performance test scores with "tissue concentration" of lysergic acid diethylamide in human subjects. Clin Pharmacol Ther. 1968 Sep-Oct;9(5):635–638. doi: 10.1002/cpt196895635. [DOI] [PubMed] [Google Scholar]


