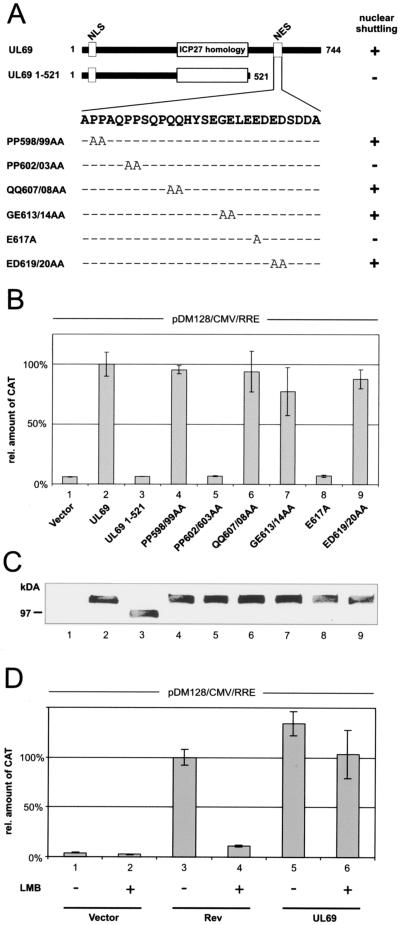
FIG. 2.
pUL69-mediated RNA export requires nucleocytoplasmic shuttling but is CRM1 independent. (A) Schematic representation of the wt UL69 protein, a C-terminal deletion mutant lacking the NES (UL69 1-521), and a series of pUL69 alanine replacement mutants carrying point mutations within the NES. The region of highest homology to the HSV-1 ICP27 and the location of the nuclear import signal (NLS) and the NES are depicted for pUL69. + or − indicates the nucleocytoplasmic shuttling activity of each pUL69 mutant as determined by heterokaryon analysis (34). (B) CAT protein expression was measured in HeLa cell lysates cotransfected with pDM128/CMV/RRE and plasmids encoding the proteins depicted in panel A. The values are given as percentages of the amount of CAT protein detected with wt UL69; 100% represents a 10.4-fold increase of CAT protein in comparison to basal levels. (C) Western blot analysis of HeLa cell extracts after transfection of the pUL69 mutants indicated in panel A using a polyclonal antiserum specific for pUL69. (D) Relative CAT protein amount expressed in HeLa cells transfected with pDM128/CMV/RRE and plasmids expressing Rev or pUL69 as indicated. As indicated by a + sign cells were washed with fresh medium supplemented with 2.5 ng/ml LMB at 12 h posttransfection and incubated for a further 6 h. At this point cell extracts were made and CAT protein was quantified. The results are expressed as the amount of CAT relative to the amount of CAT protein expressed in the presence of Rev.