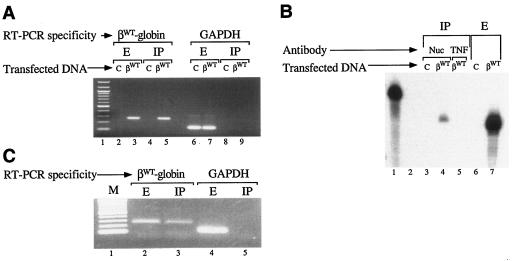
FIG. 5.
Nucleolin binds to β-globin mRNA in intact cells. (A, B) Specificity of nucleolin-β-globin mRNA interaction in vivo. (A) HeLatTA cells were transfected with pTRE-βWT (βWT) or with an empty pTRE vector control (C). Total RNA recovered from cell extract (E) or nucleolin immunoprecipitate (IP) was RT-PCR amplified using βWT sequence-specific oligomers, generating a 261-bp product (lanes 2 to 5), or with GAPDH mRNA-specific oligomers, producing a 116-bp product (lanes 6 to 9). Lane 1 contains a 100-bp DNA ladder. (B) Total RNA was recovered from immunoprecipitate (lanes 3 to 5) or extract (lanes 6 and 7) prepared from cells transfected with pTRE-βWT (βWT) or with the empty pTRE vector control (C). Immunoprecipitates were prepared using nucleolin- or tumor necrosis factor-specific antibodies (Nuc or TNF, respectively). RNAs were analyzed by RNase protection using in vitro-transcribed, 32P-labeled RNA probes (84). Intact and RNase-digested 32P-labeled probes were run in lanes 1 and 2, respectively. (C) Nucleolin binds β-globin mRNA in intact human erythroid cells. Purified RNA prepared from the extract or nucleolin immunoprecipitate of density-fractionated human erythroid cells was RT-PCR amplified using human β-globin- and GAPDH-specific oligomers. M, DNA size markers.