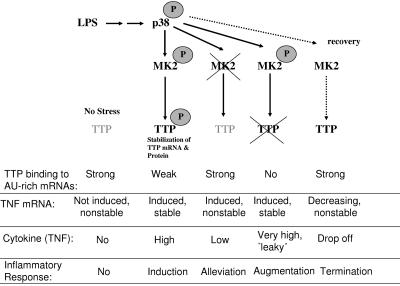
FIG. 8.
Model of posttranscriptional regulation by the p38/MK2/TTP pathway in response to LPS in the different genetic situations. TTP is expressed in small amounts in nonstimulated cells (no stress), is not phosphorylated, and is able to bind and destabilize rare TNF mRNA occasionally transcribed and, hence, elevates the threshold of inflammation. In the case of stimulation by LPS, the rate of TNF and TTP transcription increases, and MK2 gets activated, phosphorylates TTP, and reduces its binding to ARE, making ARE-containing mRNA stable. Phosphorylated TTP protein is stable, and its accumulation can be important in the recovery step, when MK2 is no longer active and TTP is rapidly dephosphorylated. Dephosphorylated TTP binds to and destabilizes TNF mRNA, resulting in decreasing expression of TNF. Apparently, in MK2-deficient cells, codegradation of nonphosphorylated TTP bound to TNF mRNA becomes the default pathway, resulting in low levels of both components, and causes alleviation of inflammatory response. In contrast, TTP-deficient mice which are compromised in degradation of ARE-containing mRNAs produce increased levels of TNF upon LPS stimulation and, even in the absence of stimulation, develop TNF excess syndrome. Since TTP is downstream of MK2, TNF excess syndrome is not modulated by MK2 deficiency in the absence of TTP.